Abstract
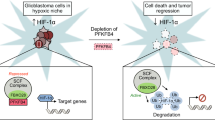
Alterations in both cell metabolism and transcriptional programs are hallmarks of cancer that sustain rapid proliferation and metastasis1. However, the mechanisms that control the interaction between metabolic reprogramming and transcriptional regulation remain unclear. Here we show that the metabolic enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) regulates transcriptional reprogramming by activating the oncogenic steroid receptor coactivator-3 (SRC-3). We used a kinome-wide RNA interference-based screening method to identify potential kinases that modulate the intrinsic SRC-3 transcriptional response. PFKFB4, a regulatory enzyme that synthesizes a potent stimulator of glycolysis2, is found to be a robust stimulator of SRC-3 that coregulates oestrogen receptor. PFKFB4 phosphorylates SRC-3 at serine 857 and enhances its transcriptional activity, whereas either suppression of PFKFB4 or ectopic expression of a phosphorylation-deficient Ser857Ala mutant SRC-3 abolishes the SRC-3-mediated transcriptional output. Functionally, PFKFB4-driven SRC-3 activation drives glucose flux towards the pentose phosphate pathway and enables purine synthesis by transcriptionally upregulating the expression of the enzyme transketolase. In addition, the two enzymes adenosine monophosphate deaminase-1 (AMPD1) and xanthine dehydrogenase (XDH), which are involved in purine metabolism, were identified as SRC-3 targets that may or may not be directly involved in purine synthesis. Mechanistically, phosphorylation of SRC-3 at Ser857 increases its interaction with the transcription factor ATF4 by stabilizing the recruitment of SRC-3 and ATF4 to target gene promoters. Ablation of SRC-3 or PFKFB4 suppresses breast tumour growth in mice and prevents metastasis to the lung from an orthotopic setting, as does Ser857Ala-mutant SRC-3. PFKFB4 and phosphorylated SRC-3 levels are increased and correlate in oestrogen receptor-positive tumours, whereas, in patients with the basal subtype, PFKFB4 and SRC-3 drive a common protein signature that correlates with the poor survival of patients with breast cancer. These findings suggest that the Warburg pathway enzyme PFKFB4 acts as a molecular fulcrum that couples sugar metabolism to transcriptional activation by stimulating SRC-3 to promote aggressive metastatic tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Dang, C. V. Cancer cell metabolism: there is no ROS for the weary. Cancer Discov. 2, 304–307 (2012).
Gojis, O. et al. The role of SRC-3 in human breast cancer. Nat. Rev. Clin. Oncol. 7, 83–89 (2010).
Anzick, S. L. et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277, 965–968 (1997).
Xu, J., Wu, R. C. & O’Malley, B. W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 9, 615–630 (2009).
Han, S. J., Lonard, D. M. & O’Malley, B. W. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol. Metab. 20, 8–15 (2009).
Wu, R. C. et al. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol. Cell 15, 937–949 (2004).
Wu, R. C., Feng, Q., Lonard, D. M. & O’Malley, B. W. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129, 1125–1140 (2007).
Dasgupta, S., Lonard, D. M. & O’Malley, B. W. Nuclear receptor coactivators: master regulators of human health and disease. Annu. Rev. Med. 65, 279–292 (2014).
Lonard, D. M. & O’Malley, B. W. The expanding cosmos of nuclear receptor coactivators. Cell 125, 411–414 (2006).
Dasgupta, S. & O’Malley, B. W. Transcriptional coregulators: emerging roles of SRC family of coactivators in disease pathology. J. Mol. Endocrinol. 53, R47–R59 (2014).
Lonard, D. M., Nawaz, Z., Smith, C. L. & O’Malley, B. W. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol. Cell 5, 939–948 (2000).
Yi, P. et al. Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol. Cell 29, 465–476 (2008).
Pilkis, S. J., Claus, T. H., Kurland, I. J. & Lange, A. J. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu. Rev. Biochem. 64, 799–835 (1995).
Rousseau, G. G. & Hue, L. Mammalian 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a bifunctional enzyme that controls glycolysis. Prog. Nucleic Acid Res. Mol. Biol. 45, 99–127 (1993).
Chesney, J. et al. Fructose-2,6-bisphosphate synthesis by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) is required for the glycolytic response to hypoxia and tumor growth. Oncotarget 5, 6670–6686 (2014).
Chen, H. et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90, 569–580 (1997).
Colosia, A. D. et al. Induction of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase mRNA by refeeding and insulin. J. Biol. Chem. 263, 18669–18677 (1988).
Zhang, C. S. et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116 (2017).
Jiang, S. et al. Scaffold attachment factor SAFB1 suppresses estrogen receptor α-mediated transcription in part via interaction with nuclear receptor corepressor. Mol. Endocrinol. 20, 311–320 (2006).
Stashi, E. et al. SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Reports 6, 633–645 (2014).
DeBerardinis, R. J., Sayed, N., Ditsworth, D. & Thompson, C. B. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 18, 54–61 (2008).
Nikolai, B. C. et al. HER2 signaling drives DNA anabolism and proliferation through SRC-3 phosphorylation and E2F1-regulated genes. Cancer Res. 76, 1463–1475 (2016).
Patra, K. C. & Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 39, 347–354 (2014).
Li, L., Zhou, X., Ching, W. K. & Wang, P. Predicting enzyme targets for cancer drugs by profiling human metabolic reactions in NCI-60 cell lines. BMC Bioinformatics 11, 501 (2010).
Zhu, B. et al. A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metab. 25, 1305–1319.e9 (2017).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Long, W. et al. ERK3 signals through SRC-3 coactivator to promote human lung cancer cell invasion. J. Clin. Invest. 122, 1869–1880 (2012).
Dasgupta, S. et al. Coactivator SRC-2-dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J. Clin. Invest. 125, 1174–1188 (2015).
Wang, L. et al. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell 28, 240–252 (2015).
Jin, F. et al. A novel [15N]glutamine flux using LC-MS/MS-SRM for determination of nucleosides and nucleobases. J. Anal. Bioanal. Tech. 6, 267 (2015).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Stadler, C. et al. Immunofluorescence and fluorescent-protein tagging show high correlation for protein localization in mammalian cells. Nat. Methods 10, 315–323 (2013).
Foulds, C. E. et al. Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics. Mol. Cell 51, 185–199 (2013).
Welte, T. et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 18, 632–644 (2016).
Ciriello, G. et al. Comprehensive Molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Acknowledgements
We would like to thank R. DeBerardinis, K. Gurova and M. Nikiforov for discussions and suggestions. We thank the core facilities at BCM: Tissue Culture Core, CBASS core, Pathology Core, Proteomics Core (NIH P30CA125123), Metabolomics (CPRIT RP170005), and Gene Vector Core. We also acknowledge U.-I. Chen for technical assistance and Cell Signaling Technology for the p-SRC-3-Ser857 antibody. This work was supported by funds from the Susan G. Komen (PG12221410 to B.W.O. and PDF14300468 to S.D.), the National Institutes of Health (NIH; 4R01HD007857, HD08818 and 4P01DK059820 to B.W.O.; 1K22CA207578 to S.D. and R01CA220297 to N.P.), DOD (W81XWH-13-1-0285 to B.W.O. and W81XWH-16-1-0297 to S.D.), American Cancer Society 127430-RSG-15-105-01-CNE to N.P., and P30CA016056 to the Roswell Park Comprehensive Cancer Center.
Reviewer information
Nature thanks F. Böhmer, J. Carroll and C. Frezza for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
S.D. and B.W.O. conceived the project. S.D. performed most experiments with the following exceptions: N.P. independently performed metabolic profiling and isotope-tracing analysis, J.M.C. and S.Y.J. independently performed proteomics analysis and identified the phosphorylation site, and B.Z. analysed the cistromes and motif analysis. K.R. performed all the data analysis, statistical tests and human datasets for clinical correlation under the supervision of C.C. B.C.N. developed plasmids used in the study; C.E.F. validated the pSRC-3-Ser857 antibody, and P.Y. purified full-length SRC-3 protein and recombinant SRC-3 fragments. T.F.W. provided the RNAi-kinome library and supervised the library screen. X.H.-F.Z. supervised the animal study. B.W.O., M.-J.T. and S.Y.T. supervised the overall project. S.D. and B.W.O. wrote the manuscript with feedback from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Kinome-wide screen identified potential kinases regulating SRC-3 intrinsic transcriptional activity.
a, HeLa cells expressing varying concentrations of the pBIND or pBIND-SRC-3 constructs were used to measure SRC-3 activity. n = 4 biologically independent samples. *P < 0.000001, one-way ANOVA with Sidak’s multiple comparison test. RLU are normalized by protein content. b, HeLa cells expressing pBIND or pBIND-SRC-3 were treated with siRNA targeting GFP or PRKCZ at the indicated dose followed by luciferase assay to measure SRC-3 activity. n = 3 biologically independent samples. *P < 0.000001, one-way ANOVA with Tukey’s multiple comparison test. c, Different control siRNAs targeting GFP or luciferase (Luc) were used to measure SRC-3 activity in HeLa cells expressing pBIND or pBIND-SRC-3. n = 3 biologically independent samples. The GFP control siRNAs in the red box were used in the library screen as controls. d, Effect on SRC-3 transcriptional activity by three sets of siRNA (sets A, B and C) targeting 636 human kinases in HeLa cells. Effect of GFP control siRNA was set at 1 (dotted line), the cut-off fold for increased activation was set at 2, and reduced activity at 0.75 following z-score analysis. n = 3 siRNAs per /kinase, n = 6 siGFP per plate; total n = 1,908 (siRNAs targeting kinases) n = 144 (siGFP control) independent samples. e, SRC-3 activity in HeLa cells across 24 kinome-library plates in the presence of control siRNA targeting GFP. n = 6 biologically independent replicates for each plate. f, A secondary screen was performed in HeLa cells to confirm the primary screen hits using a pooled siRNA targeting the kinases followed by SRC-3 transcriptional activity. n = 3 biologically independent samples. Boxes are as in Fig. 2e. g, Relative proliferation of MDA-MB-231 cells 4 days after treatment with siRNAs targeting GFP (control), SRC-3 or the indicated kinases. n = 3 biological replicates. *P < 0.0001. two-way ANOVA with Dunnett’s multiple comparisons test. Unless stated otherwise, data are mean ± s.d.
Extended Data Fig. 2 PFKFB4, the top hit from the kinase screen, enhances the transcriptional activity of SRC-3.
a, Effect of PFKFB4 knockdown on SRC-3 transcriptional activity in various breast cancer cell lines. n = 3 or n = 4 (siGFP plus pBIND-SRC-3) biologically independent cells. *P < 0.000009, two-way ANOVA with Tukey’s multiple comparison test. b, SRC-3 transcriptional activity in MDA-MB-231 cells expressing shRNAs targeting PFKFB4 (#09 and #20) or non-targeting control, co-transfected with pBIND or pBIND-SRC-3. n = 5, biological replicates. *P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons test. c, Protein expression of PFKFB4 or actin in MDA-MB-231 cells expressing shRNAs targeting PFKFB4. d, Expression of PFKFB4 and SRC-3 mRNA in indicated breast tumour cells after treatment with siRNAs targeting GFP control or PFKFB4. n = 4 or n = 3 biological replicates. See Source Data for exact P values. e, Expression of PFKFB4 and SRC-3 mRNA in MDA-MB-231 cells transduced with adenoviruses expressing GFP or PFKFB4. n = 6 biologically independent cells. ***P < 0.000001, two-way ANOVA with Tukey’s multiple comparison test. f, Left, MDA-MB-231 cells were stained with specific antibodies against SRC-3 (rabbit) and PFKFB4 (mouse) before proximity ligation assay (PLA). The PLA signals between endogenous SRC-3 and PFKFB4 are shown in the red channel, DAPI was used to stain the nuclei (blue) and the merge images show the overlay of the red and blue channels. Two representative fields from biologically independent experiments were shown from n = 5. Right, control cells were stained with either one of the antibodies against SRC-3, PFKFB4 or secondary antibody-conjugated with probes. Scale bars, 20 μm (left), 40 μm (right). Data are representative of three biologically independent experiments with similar results, and are shown as mean ± s.d.
Extended Data Fig. 3 PFKFB4 functions as a protein kinase by phosphorylating SRC-3 at the Ser857 residue.
a, In vitro PFKFB4 kinase assay in the presence of purified SRC-3 protein, F6P, ATP and increasing concentration of recombinant PFKFB4 enzyme followed by SDS–PAGE. Immunoblotting with pSer/Thr antibody shows the level of phosphorylated SRC-3 protein. b, In vitro PFKFB4 kinase assay in presence of purified SRC-3 protein, PFKFB4 enzyme and varying concentrations of F6P and ATP followed by SDS–PAGE. Immunoblotting with pSer/Thr antibody shows the level of pSRC-3 protein. c, Coomassie blue stain showing the levels of GST-fused SRC-3 fragments used in in vitro kinase reactions performed in Fig. 2b. d, Proteomics analysis of in vitro kinase assay using the GST–SRC-3-CID fragment in the presence of PFKFB4 enzyme and ATP followed by mass spectrometric analyses. Mass spectrum shows the green phosphorylation peak. e, Proteomics analysis of an in vitro kinase assay using a Ser857Ala-mutated GST–SRC-3-CID protein in the presence of PFKFB4 enzyme and ATP, followed by mass spectrometric analyses. Mass spectrum failed to detect phosphorylation peaks in the Ser857Ala-mutated SRC-3-CID protein. f, Expression of PFKFB1, PFKFB2, PFKFB3 and PFKFB4 in MDA-MB-231 cells expressing shRNAs targeting PFKFB4 (#09 and #20). mRNA levels were normalized to internal housekeeping gene ACTB. n = 3 biological replicates. *P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test. g, Protein levels of pSRC-3-Ser857, total-SRC-3 and actin in MDA-MB-231 cells stably expressing non-targeting control shRNA, SRC-3 shRNA, or SRC-3 shRNA plus the shRNA-resistant Ser857Ala SRC-3 mutant (shSRC-3 + S857A) or SRC-3 shRNA plus wild-type SRC-3 (shSRC-3 + WT-SRC-3) cultured in 25 mM glucose. Protein bands were quantified by ImageJ after normalization to β-actin. h, MDA-MB-231 cells stably expressing non-targeting shRNA or shRNA targeting PFKFB4 were grown in the presence of 25 mM glucose or were glucose-starved for 4 h followed by incubation with streptolysin O for 5 min. FBP (10 μM) was added to glucose-starved cells for an additional 1 h, followed by cell lysis and immunoblotting. Protein bands were quantified by ImageJ after normalization to β-actin and the non-targeting shRNA lane was set to 1. i, Relative luciferase activity showing the transcriptional activity of SRC-3 in MDA-MB-231 cells transduced with adenoviruses expressing GFP or PFKFB4 cultured in the presence of 5 mM, 15 mM or 25 mM glucose. n = 6 (pBIND) and n = 3 (pBIND-SRC-3) biological cell samples. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. Data in a–c, f–h are representative of three biologically independent experiments with similar results, and in d, e are representative of two biologically independent experiments each run with three different reactions all showing similar results and peptide coverage. Data are mean ± s.d.
Extended Data Fig. 4 Ser857 phosphorylation enhances SRC-3 transcriptional activity.
a, Relative luciferase activity showing the activity of wild-type SRC-3, and the Ser857Ala and Ser857Glu SRC-3 mutants in MDA-MB-231 cells transduced with lentivirus expressing non-targeting shRNA or PFKFB4 shRNA cultured in the presence of 5 mM or 25 mM glucose. n = 3 biological cell samples. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. b, Relative luciferase activity showing the activity of SRC-3 in MDA-MB-231 cells stably expressing lentivirus PFKFB4 shRNA and cultured in the presence of 25 mM glucose. The cells are then co-transfected with empty vector, wild-type PFKFB4 and PFKFB4 mutants Gly46Ala, Pro48Ala, Gly51Ala, Arg230Ala and Arg238Ala. n = 6 biological cell samples. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. c, MDA-MB-231 cells stably expressing shRNAs targeting PFKFB4 (231-shPFKFB4) were transfected with constructs expressing empty vector (vector), wild-type PFKFB4, and PFKFB4 mutants Gly46Ala, Pro48Ala, Gly51Ala, Arg230Ala and Arg238Ala, and cultured in presence of 25 mM glucose. Protein levels of pSRC-3-Ser857, PFKFB4 and β-actin were detected by immunoblotting. d, Relative luciferase activity showing the activity of oestrogen receptor-α (ERα) in MCF7-Mar-luc cells transduced with lentivirus expressing non-targeting shRNA or SRC-3 shRNA cultured in the presence of 5 mM or 25 mM glucose stimulated with 100 nm E2, or with ethanol control (−E2). n = 3 biological cell samples. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. e, Relative luciferase activity showing the activity of ERα in MCF7-Mar-luc cells transduced with adenovirus expressing GFP or PFKFB4. Cells transduced with PFKFB4 adenovirus were infected with non-targeting shRNA or SRC-3 shRNA after 2 days and then cultured in the presence of 5 mM or 25 mM glucose stimulated with ethanol (−E2) or with 100 nM E2. n = 3 biological cell samples. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. f, Survival assay in MCF7 cells showing the effect of non-targeting shRNA, SRC-3 shRNA, and re-expression of wild-type SRC-3 or SRC-3(Ser857Ala) mutant in SRC-3-depleted cells cultured in charcoal-stripped medium supplemented with 25 mM glucose and E2 for 7 days. n = 3 biological independent data are shown. All data are representative of three independent experiments with similar results, and shown as mean ± s.d.
Extended Data Fig. 5 Increased glucose and purines are required for SRC-3-dependent growth.
a, Real-time measurement of MCF10A cell proliferation transduced with adenoviruses expressing GFP or SRC-3 in the presence of 93 different metabolites. n = 3 independent plates run for each sample. b, Relative growth of MCF10A cells transduced with adenoviruses expressing GFP or SRC-3 in the presence of d-glucose (b), adenosine (c) and inosine (d). n = 6 biological cell samples. **P < 0.01, ***P < 0.001, ****P < 0.0001, unpaired t-test two tailed. Boxes are as in Fig. 2e. e, f, Relative levels of intermediary metabolites in MDA-MB-231 cells after treatment with shRNAs targeting PFKFB4 or SRC-3 compared to control shRNA. e, Glycolytic and PPP metabolites. f, Nucleotides. n = 3 biological independent samples. *P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test. g, Total levels of purines in MCF10A cells transduced with with adenoviruses expressing GFP or SRC-3. n = 3 biological independent samples. *P < 0.05, ***P < 0.001, two-way ANOVA with Tukey’s multiple comparisons test. See Source Data for exact P values. Unless stated otherwise, data are mean ± s.d.
Extended Data Fig. 6 SRC-3 drives the purine synthesis program under conditions of active glycolysis.
a, MDA-MB231 cells stably expressing control shRNA, PFKFB4 shRNA and SRC-3 shRNA were fed with [6-13C]glucose. Ribulose/xylulose-5P (m + 1) labelling from [6-13C]glucose is shown. n = 3 biological cell samples. ***P = 0.00013, ****P = 0.000078, one-way ANOVA with Tukey’s multiple comparisons test. b, Genes involved in oxidative and non-oxidative PPP. n = 3 biological cell samples. *P = 0.0431, two-way ANOVA with Sidak’s multiple comparisons test. c, Genes involved in nucleotide synthesis. n = 3 biological cell samples. *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA with Sidak’s multiple comparisons test. d, mRNA expression of the metabolic enzymes TKT, XDH and AMPD1 in MDA-MB-231 cells transduced with adenovirus expressing GFP (control) and PFKFB4 cultured in the presence of 5 mM, 15 mM or 25 mM glucose. n = 3 biological cell samples. **P < 0.01, ***P < 0.001, ****P = 0.0001, two-way ANOVA with Dunnett’s multiple comparisons test. e, f, MDA-MB231 cells stably expressing control shRNA, PFKFB4 shRNA and SRC-3 shRNA were fed with [6-13C]glucose. Seduheptulose-7P (m + 1) (e) and erythrose-4P (f) labelling from [6-13C]glucose are shown. n = 3 biological cell samples. **P < 0.01, ****P = 0.001, two-way ANOVA with Dunnett’s multiple comparisons test (e) or with Tukey’s multiple comparison test (f). Boxes are as in Fig. 2e. See Source Data for exact P values. Unless stated otherwise, data are mean ± s.d.
Extended Data Fig. 7 Growth defect due to loss of SRC-3 or PFKFB4 is rescued by exogenous purines.
a, Expression of the metabolic enzymes encoded by TKT, XDH, AMPD1 and SRC-3 in MDA-MB-231 cells expressing control shRNA, SRC-3 shRNA or SRC-3 shRNA plus re-expression of shRNA-resistant wild-type SRC-3 protein (shSRC-3-21 + WT-SRC-3). n = 4 biological cell samples. *P < 0.05, **P < 0.01, ***P < 0.001, ****P = 0.0001, two-way ANOVA with Tukey’s multiple comparisons test. b, Relative proliferation of MDA-MB-231 cells expressing shRNA targeting SRC-3 (shSRC-3#01 and shSRC-3#02) or non-targeting control shRNA after treatment with siRNAs targeting luciferase (siLuc; as a control) or PFKFB4 under the conditions indicated. n = 6 samples from biologically independent experiments. ****P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. c, MDA-MB231 cells stably expressing control shRNA, PFKFB4 shRNA or SRC-3 shRNA were fed with [U-13C]glucose for 48 h. Adenosine 13C-labelling from [U-13C]glucose is shown. n = 3 samples from biologically independent experiments. one-way ANOVA with Tukey’s multiple comparisons test. Boxes are as in Fig. 2e. Data are representative of three biologically independent experiments with similar results. See Source Data for exact P values. Unless stated otherwise, data are mean ± s.d.
Extended Data Fig. 8 PFKFB4–SRC-3 stabilizes ATF4 transcription factor to promote purine synthesis.
a, Chromatin localization peaks of SRC-3 and ATF4 on Tkt, Xdh and Ampd1 genes in mouse liver. b, ATF4-binding peaks are conserved on three SRC-3 target purine biosynthetic genes in both mouse and human genomes. c, Chromatin immunoprecipitation (ChIP) of ATF4, total SRC-3 and pSRC-3-Ser857 from MDA-MB-231 cells treated with 5 mM or 25 mM glucose compared to an IgG isotype control. qPCR was performed to determine amount of promoter enrichment. d, ChIP–qPCR was performed from MDA-MB-231 cells cultured in 25 mM glucose expressing SRC-3 shRNA, PFKFB4 shRNA or control shRNA. n = 3 biological cell samples. *P < 0.01, **P < 0.0001, ***P < 0.00005, ****P < 0.000001, one-way ANOVA with Tukey’s multiple comparisons test compared to 5 mM glucose groups (c) and compared to NT shRNA group (d). e, ChIP of ATF4, total SRC-3 (BD Biosciences antibody), and pSRC-3-Ser857 from MDA-MB-231 cells on the AMPD1 promoter treated with non-targeting siRNA or siRNA against ATF4, and cultured in presence of 25 mM glucose compared to an IgG isotype control. qPCR was performed to determine the amount of promoter enrichment. n = 3 biological cell samples. ***P < 0.001, ****P < 0.000001, one-way ANOVA with Tukey’s multiple comparisons test. f, mRNA expression of TKT, XDH, AMPD1 and SRC-3 in MDA-MB-231 cells expressing siRNA targeting control or ATF4 siRNA. n = 3 biological cell samples. two-way ANOVA with Sidak’s multiple comparisons test. See Source Data for exact P values. Data are mean ± s.d.
Extended Data Fig. 9 The PFKFB4–SRC-3 axis promotes breast tumour growth and metastasis.
a, Primary tumours resected out after 6 weeks. b, Ki67 staining of primary tumours from animals injected with MDA-MB-231 cells stably expressing control shRNA, SRC-3 shRNA or PFKFB4 shRNA. Data are representative of five fields per slide from n = 5 animals per group with similar findings. Scale bar, 100 μm. c, Quantification of Ki67-positive cells in the tumour. n = 5 animals per group, average of five fields counted from each slide. ****P = 0.0001, one-way ANOVA with Dunnett’s multiple comparisons test. d, Primary tumour growth in animals injected with MDA-MB-231 cells stably expressing shRNA targeting SRC-3, PFKFB4, or expression of wild-type SRC-3 or the Ser857Ala mutant in the SRC-3-depleted cells. n = 5 animals per group. *P < 0.000001, two-way ANOVA with Tukey’s multiple comparisons test. e, Immunoblot showing the relative expression of SRC-3 in primary tumours from MDA-MB-231 cells stably expressing control shRNA, SRC-3 shRNA, or after re-expression of wild-type SRC-3 or the Ser857Ala mutant in the SRC-3-depleted cells. n = 5 animals per group was pooled to generate the tumour lysate used for analysis. f, Graph representing the photon flux of animals from different groups. n = 5 animals for wild-type SRC-3, the Ser857Ala mutant and PFKFB4 shRNA, and n = 4 animals for SRC-3 shRNA. *P < 0.0001, one-way ANOVA with Dunnett’s multiple comparisons test. Line shows median with range. g, mRNA expression of three metabolic enzymes (TKT, XDH and AMPD1), SRC-3 and PFKFB4 from the primary tumours. n = 5 animals per group. **P < 0.05, ***P < 0.001, ****P = 0.0001, two-way ANOVA with Tukey’s multiple comparisons test. h, Expression of PFKFB4 in patients with breast cancer across different subtypes compared to normal breast tissue. Normal basal = 17; basal = 139; normal_Her2 = 9; Her2 = 67; normal luminal A = 62; luminal A = 418; normal luminal B = 21 and LumB = 186. Line in the centre of the rectangle represents the median, top edge of the rectangle represents the third quartile, bottom edge of the rectangle represents the first quartile, top whisker represents the maximum and bottom whisker represents the minimum. All data are representative of three biologically independent experiments with similar results, and are shown as mean ± s.d. unless otherwise stated. See Source Data for exact P values.
Extended Data Fig. 10 The PFKFB4–SRC-3 axis drives transcriptional programming in patients with breast cancer.
a, b, Expression of pSRC-3, SRC-3 and PFKFB4 in ER+ breast tumour specimens and matched adjoining normal tissues as detected by immunoblotting. n = 14 patients with ER+ breast cancer. c, Semi-quantitative levels of bands shown in a and b, analysed by densitometry using UVP Vision Works LS software, and normalized relative to actin to calculate the fold change (tumour/normal) and plotted to obtain the correlation between PFKFB4 and pSRC-3-Ser857 expression. n = 14 normal and tumour tissues. R = 0.63, P = 0.02 Spearman’s rank correlation coefficient. d, log fold change in protein expression of the PFKFB4–SRC-3 signature compared to the control knockdown (non-targeting shRNA) as determined using a parametric t-test as implemented in the python (spicy) statistical system. Significance P < 0.05 and fold change exceeding 1.25× were used to classify true regulators of SRC-3 activity. n = 3 biologically independent samples. e, Kaplan–Meier survival plot showing poor survival of patients with breast cancer with basal subtype (triple-negative) disease exhibiting an increased expression of a common proteomic signature induced by the PFKFB4 and SRC-3 axis. The cohort of patients was collected by the TCGA. P = 0.0365, log-rank test; P = 0.02971, Cox proportional hazards, two-sided. f, Cartoon model describing the crosstalk between glycolysis and purine generation highlighting the essential steps regulated by pSRC-3-Ser857. This PFKFB4-dependent SRC-3 phosphorylation enhances mRNA expression of genes involved in purine metabolism driving breast tumour growth, proliferation and metastasis. AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; AMP, adenosine monophosphate; F1,6-P, fructose 1,6 bisphosphate; IMP, inosine monophosphate. g, Model showing that, in glycolytic breast tumours, activated PFKFB4 drives SRC-3 phosphorylation at Ser857, which then activates ER-positive primary tumour growth in conjunction with E2-liganded ER, as well in ER-negative/recurrent tumours in conjunction with ATF4, driving aggressive metastatic disease. Data are mean ± s.d.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3 and the uncropped gel source data
Source data
Rights and permissions
About this article
Cite this article
Dasgupta, S., Rajapakshe, K., Zhu, B. et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature 556, 249–254 (2018). https://doi.org/10.1038/s41586-018-0018-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0018-1
This article is cited by
-
Fructose-1,6-bisphosphatase 1 dephosphorylates and inhibits TERT for tumor suppression
Nature Chemical Biology (2024)
-
Visualization of breast cancer-related protein synthesis from the perspective of bibliometric analysis
European Journal of Medical Research (2023)
-
Comparative clinical significance and biological roles of PFKFB family members in oral squamous cell carcinoma
Cancer Cell International (2023)
-
Anoikis resistance––protagonists of breast cancer cells survive and metastasize after ECM detachment
Cell Communication and Signaling (2023)
-
THOC3 interacts with YBX1 to promote lung squamous cell carcinoma progression through PFKFB4 mRNA modification
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.