Abstract
Mg11B2 has a great application prospect in the superconducting coils for fusion reactor as the “low activation superconductors”. The un-doped Mg11B2 and Cu-doped Mg11B2 bulks using 11B as a boron precursor were fabricated by low-temperature sintering in present work. It was found that the prepared Mg11B2 low activation superconductors exhibit better Jc performance than all of other Mg11B2 samples reported in previous studies. As for Cu doped Mg11B2, minor Cu addition can obviously improve the Mg11B2 grain crystallization and reduce the amount of MgO impurity. Hence, improved grain connectivity and higher Jc at low fields is obtained in Cu doped Mg11B2 samples. For un-doped samples, refined grains and more MgO impurity with proper size brought about more flux pinning centers, resulting in better Jc performance at high fields.
Similar content being viewed by others
Introduction
The observation of superconductivity in MgB2 at 39 K in 20011 has attracted lots of attention. MgB2 shows excellent properties such as simple crystal structure, large coherence length, relatively high critical current density, and transparency of the grain boundaries and relatively low cost material. Using kinds of approaches, MgB22,3,4,5,6,7,8,9 has been fabricated into bulks, single crystals, thin films, tapes and wires in various methods10,11. Taken together, these properties and preparation techniques support MgB2 to be a promising candidate for important large-scale and electronic device applications.
The radioactivity decay time of all kinds of superconducting wire12 who had broad application prospect in the international thermonuclear experimental reactor (ITER) Tokamak superconducting coil showed that Nb-based superconductor wires who have been widely used nowadays had a very long induction of radioactive decay time, so Nb-based superconductor wires needed much longer time to cool down. The longer cooling time brought negative impact on the environment and treating processes. Furthermore, despite their relatively high Jcs13,14,15, Nb-based superconductors suffer high operation costs due to the usage of liquid helium as coolant. In contrast, MgB2 radioactivity decay time is very short and has higher running temperature, so it has a great application prospect in the fusion reactor as the “low activation superconductors”. In general, the natural elementary boron is composed of 20% 10B and 80% 11B. In the irradiation environment, the reaction 10B + n → 7Li + He (gas) will occur due to the large thermal neutron capture cross section of 10B. Hence, as part of superconductor, there is no guarantee for the long-term stability of the MgB2 superconducting magnet. Isotope research shows that 11B is much more stable than 10B in neutron irradiation environment due to the smaller neutron capture cross section16 which can ensure the operation stability of MgB2 superconductor magnet. Previous studies have been focused on the theoretical calculation of isotope boron effect on the MgB217,18, but little on the experiment research. Only a few previous experimental studies put many emphases on the performance of the Mg11B2 superconducting wires19,20, which performed obviously different from the traditional MgB2 wire with natural B powder, and the critical current density exhibits much lower than the traditional MgB2 with natural B powder20.
On the other hand, one can know that the traditional MgB2 samples using natural boron powder which fabricated at low temperature generally had higher critical current density at high fields than those prepared at high temperature due to inferior crystallinity21,22,23. However, the reaction at low temperature generally took a very long time to form the complete MgB2 because of the low diffusion rate of atom in the solid-state below the melting point of Mg24. Y. Hishinuma et al. also reported that the successful synthesis of Mg11B2 samples with higher Jc performance needs to take as long as 200 h by heating at 500 °C20. From this view, it makes sense to rapidly fabricate Mg11B2 samples with improved Jc by heating at low temperature.
In previous studies, the influence of Cu addition on the sintering process and superconducting properties of MgB2 samples with natural boron powder were systemically studied25,26 and it was found that minor Cu addition was proved to benefit to the formation of MgB2 phase at low temperature, and thus MgB2 bulks with excellent Jc performance can be rapidly fabricated at low temperature by Cu activated sintering26,27. Inspired by this, herein the influence of Cu addition on the Mg11B2 bulks with isotope 11B powder will be studied and Cu addition is expected to benefit to its low temperature sintering process as well as superconducting properties. Actually, the research about the influence mechanism of Cu addition on the Mg11B2 bulks is seldom carried out till now.
Based on these backgrounds, in present study, we focused on the synthesis of Mg11B2 with higher superconducting properties by heating at low temperature for short holding time. In this study pure 11B was used as the raw material instead of natural B, with the purpose of solving the tokamak plasma problem in controllable thermonuclear fusion. Besides the un-doped samples, we also investigated the phase formation and superconducting properties in the Cu-doped Mg11B2 samples. Combined with the measurement of the Tc and critical current density, the effects of the element 11B and Cu addition on the superconducting properties of MgB2 are concluded.
Experimental
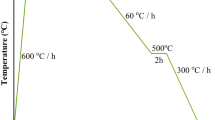
The samples of un-doped Mg11B2 and Mg11B2 + 5wt.% Cu were prepared by a solid-state sintering at low temperature. 11B powders (Amorphous, about 5 μm in size, from Pavezyum Kimya, Turkey), Mg powders (99.5% purity, 100 μm in size), and Cu powders (99.7% purity, 3 μm in size) were separately mixed in the ratio of Mg11B2 and Mg11B2 + 5wt.% Cu. Then, the mixed powders were pressed into cylindrical pellets (Ф5 × 1.5 mm). All the un-doped and Cu-doped pellets were sintered in differential thermal analysis (Mettler Toledo TGA/DSC1/) respectively at 600 °C for 5 h, 10 h and 15 h under flowing high-purity Ar gas with a heating rate of 10 °C min−1 and then cooled down to room temperature with a cooling rate of 40 °C min−1. The phase composition of the sintered samples was examined by X-ray diffractometer (XRD, Rigaku D/max 2500) using Cu Kα radiation, and the morphology of the samples was characterized by scanning electron microscope (SEM, S-4800, Hitachi). The superconducting properties were measured by superconducting quantum interference device (SQUID VSM, Quantum Design), after the samples were cut into a slab of size about 4 × 2 × 1 mm3. The corresponding Jc values were calculated from the width of magnetization hysteresis loops based on the Bean model Jc = 20ΔM/[a/(1 − a/3b)]28, where ΔM is the volume magnetization, and a and b are the sample dimensions.
Results and Discussion
Figure 1 presents the XRD patterns of the un-doped and Cu-doped Mg11B2 samples sintered at 600 °C for 5 h, 10 h and 15 h respectively. In the un-doped samples (see Fig. 1a), one can see that MgB2 is the main phase which is different from the traditional MgB2 sample with natural boron powder sintered at the same condition. In previous studies25,27, it was reported that no obvious MgB2 peaks can be observed in the un-doped MgB2 samples sintered below 650 °C only if some additional methods are employed, such as chemical doping29,30. Besides, the peaks of residual Mg phase in the un-doped samples decrease as the holding time prolongs, indicating that longer sintering time could facilitate the reaction between Mg and 11B forming Mg11B2 at low temperature. On the other hand, the residual Mg peaks cannot be recognized in all the Cu-doped samples. This implies that Cu addition can significantly promote the formation of Mg11B2 phase, just as the case of MgB2 samples with natural boron powder25,29. Local Mg-Cu liquid resulting from the eutectic reaction between Cu and Mg at about 485 °C can enhance Mg diffusion and thus accelerate the formation of MgB2 phase25,26. Furthermore, comparing with the un-doped ones, the Cu-doped samples contain less MgO impurity. The decrease of MgO impurity owes to the existence of local Mg-Cu liquid which could wrap the neighboring Mg particles and thus protect them from the oxidation31.
Based on the X-ray diffraction patterns of samples, the full width at half maximum (FWHM) was obtained and shown in Fig. 2. One can see that the FWHM of MgB2 peaks in the Cu-doped samples are smaller than that of un-doped ones. This result demonstrates that the size of MgB2 grains in the Cu-doped samples is larger than un-doped ones. In previous studies10,11,31, it was reported that the minor Cu addition can promote the growth of grain and improve the crystallization degree.
The sintering density of samples is present in Table 1. The density of all sintered samples is similar and about half of the theoretical value (2.62 g/cm3), but higher than the other ones in previous studies using the similar preparation technique32. Generally, the chemical reaction between Mg and B forming MgB2 results in about 30% volume reduction and porosity is known to be difficult to avoid during the sintering process33. This is the main reason why the in-situ sintering density of Mg11B2 bulks is generally lower than their theoretical value. In addition, the inevitable evaporation and oxidation of Mg during sintering process also have a negative impact on the sintering density. Interestingly, the sintering density of Cu-doped Mg11B2 samples was a little higher than that of un-doped samples. It could be explained that more sintering densification can occur in Cu doped samples due to the existence of local liquid sintering environment resulting from Mg-Cu liquid at low temperature. Besides, since the MgCu2 impurity as a result of Cu addition possesses relatively higher density than MgB2, finally it can contribute to the higher sintering density of Cu doped samples.
The temperature dependence of magnetization curves for the un-doped and Cu-doped Mg11B2 samples were shown in Fig. 3, and the corresponding results were listed in Table 1. The transition temperature (Tc) of Mg11B2 samples is about 36 K, which is generally lower than the normal MgB2 due to the isotope effect of 11B17,18. But the critical temperature of our Mg11B2 bulks is comparable to other Mg11B2 samples20, indicating that isotope effect is the key factor lowering Tc, rather than other processing conditions. It is worth noting that the critical temperature of all our samples is above 35 K which still meets the actual needs of practical applications. Besides, the transition width (ΔT), the interval between Tc, onset and Tc, end, becomes broad as the sintering time prolongs. As all know, the high critical temperature and sharp transition width indicate that the samples are of high crystallinity and homogeneity. It is suggested that the increased sintering time can facilitate the reaction between Mg and B completely. With extend of holding time, indefinite quantity of impurities can be introduced, which may reduce the purity and homogeneity of samples.
The critical current density (Jc) and irreversibility field (Hirr) of samples are illustrated in Fig. 4 and Table 1. The samples with Cu addition showed relatively lower Jc than the un-doped samples at high field, whereas higher Jc at low-field. Generally speaking, critical current density in MgB2 is decided by both grain connectivity and flux pinning34,35. As discussed above, minor Cu addition can obviously improve the Mg11B2 grain crystallization and reduce the amount of MgO impurity. Hence, improved grain connectivity and higher Jc at low fields is obtained in Cu doped Mg11B2 samples. On the other hand, minor Cu addition can also lead to the grain growth, implying that grain boundary pinning becomes weakened in Cu doped sample compared to undoped one. Moreover, more MgO impurity is formed in un-doped samples and some of them with proper size can also serve flux pinning centers. Consequently, better Jc performance at high fields is expected in undoped samples. From Table 1, one can see that the value of Jc and Hirr is increasing with the increasing sintering holding time. Besides, compared to previous studies20, the Jc value is higher while the sintering time is shorter due to the state of 11B which has smaller size and more activity. These advantages can accelerate the reaction rate and reduce the the raw materials exposure to oxygen, so it’s beneficial to refine the grains and get much purer samples. On the whole, the un-doped sample for 15 h possesses the optimal performance in superconductivity. In order to manifest the cause, we have accomplished the following measurement.
The SEM images of un-doped and Cu-doped Mg11B2 samples are illustrated in Fig. 5. From the SEM images, the size of MgB2 grains is larger in the Cu doped samples (about 600 ~ 800 nm) than that of un-doped samples (about 500 ~ 600 nm) and their individual grains with clear shape are easier to distinguish. In addition, the degree of crystallinity of Cu-doped samples is better than un-doped samples. These results indicate that slight Cu addition has a positive effect on the growth of grain and the improvement of crystallinity, which can enhance grain connectivity. And therefore, the Jc value of Cu-doped samples was improved at low field. The oxide particles, as the main impurities, are marked by the white arrows in these SEM images so that they can be recognized clearly. One can see that mass of oxide particles can be observed in sintered samples, especially in undoped samples. As holding time prolongs, the amount of these oxide particles gradually increase, consistent with the XRD results. It is explained that as the holding time increases, More MgO impurity can form for the reason that Mg further reacts with the trace oxygen in flowing Ar protective gas. Due to the small size of our Mg11B2 bulks (Ф5 × 1.5 mm), even the trace oxygen in the flowing Ar protective gas can result in relatively large amount of MgO impurity. Observing carefully, one can also see that the size of MgO particles became smaller and their distribution became more homogeneous with the extended holding time. It is worth noting that these small MgO particles may serve as the flux pinning centers as their size is comparable to the coherent length of pure MgB2.
Figure 6 shows the normalized pinning force F/Fp max of Mg11B2 samples in magnetic field. One can see that the pinning peaks of un-doped samples moved to high field with the increasing holding time. This result suggests that the pinning force in the un-doped samples increases as the sintering time prolongs. Combined with the results above (as shown in the Fig. 5a–c), it is explained that there are more MgO impurity with the size comparable to the coherence length of MgB2 in the un-doped samples, which is helpful to get and establish more pinning centers36.
Conclusions
In present work, the Mg11B2 samples were synthesized by low-temperature sintering. The value of Jcof un-doped samples at high fields is higher than Cu-doped ones, whereas Cu-doped samples exhibit better Jc performance at low fields. Minor Cu addition can significantly promote the growth of Mg11B2 grains as well as decrease the amount of MgO impurity. Consequently, higher sintering density and improved grain connectivity are obtained in Cu doped Mg11B2 samples, which contribute to the better Jc performance at low fields. On the other hand, larger grain size and less MgO impurity can also decrease the flux pinning centers, and thus result in the lower Jc at high fields. For un-doped samples, unconspicuous growth of grain size and more volume fraction of MgO brought about the increase of flux pinning centers, hence, the un-doped samples show higher Jc performance at high field.
Additional Information
How to cite this article: Cheng, F. et al. Improved Superconducting properties in the Mg11B2 low activation superconductor prepared by low-temperature sintering. Sci. Rep. 6, 25498; doi: 10.1038/srep25498 (2016).
References
Nakagawa, N. et al. Superconductivity at 39 K in magnesium diboride. Nature 410, 63–64 (2001).
Kim, J. H. et al. The doping effect of multiwall carbon nanotube on MgB2/Fe superconductor wire. J. Appl. Phys. 100, 013908 (2006).
Yeoh, W. K. et al. Improving flux pinning of MgB2 by carbon nanotube doping and ultrasonication. Supercond. Sci. Technol. 19, L5–L8 (2006).
Nath, M. & Parkinson, B. A. A simple sol-gel synthesis of superconducting MgB2 nanowires. Adv. Mater. 18, 1865–1868 (2006).
Jha, A. K. & Khare, N. Single-crystalline superconducting MgB2 nanowires. Supercond. Sci. Technol. 22, 075017 (2009).
Fujii, H. & Ozawa, K. Critical temperature and carbon substitution in MgB2 prepared through the decomposition of Mg(BH4)2 . Supercond. Sci. Technol. 23, 125012 (2010).
Kang, W. N. et al. MgB2 superconducting thin films with a transition temperature of 39 Kelvin. Science 292, 1521–1523 (2001).
Hossain, M. S. A. et al. The enhanced Jc and Birr of in situ MgB2 wires and tapes alloyed with C4H6O5 (malic acid) after cold high pressure densification. Supercond. Sci. Technol. 22, 095004 (2009).
Li, G. Z. et al. Critical current densities and n-values of MgB2 strands over a wide range of temperatures and fields. Supercond. Sci. Technol. 25, 025001 (2012).
Hishinuma, Y. et al. Microstructure and superconductivity of Cu addition MgB2 wires using Mg2Cu compound as additional source material. Supercond. Sci. Technol. 19, 1269–1273 (2006).
Singh, K. et al. Effect of Cu doping in MgB2 superconductor at various processing temperatures. Physica C. 450, 124–128 (2006).
Noda, T., Takeuchi, T. & Fujita, M. Induced activity of several candidate superconductor materials in a tokamak-type fusion reactor. J. Nucl. Mater. 329–333, 1590–1593 (2004).
Boutboul, T., Le-Naour, S. & Leroy, D. Critical Current Density in Superconducting Nb-Ti Strands in the 100 mT to 11 T Applied Field Range. IEEE Trans. Appl. Supercon. 17, 1184–1187 (2006).
Xu, X., Sumption, M. D. & Collings, E. W. A model for phase evolution and volume expansion in tube type Nb3Sn conductors. Supercond. Sci. Tech. 26, 075015 (2013).
Xu, X. C., Sumption, M. D. & Peng, X. Internally Oxidized Nb3Sn Strands with Fine Grain Size and High Critical Current Density. Adv. Mat. 27, 1346–1350 (2015).
Mooring, E. P., Monahan, J. E. & Huddleston, C. M. Neutron cross sections of the boron isotopes for energies between 10 and 500 keV. Nucl. Phys. 82, 16–32 (1966).
Hinks, D. G. & Jorgensen, J. D. The isotope effect and phonons in MgB2 . Physica C. 385, 98–104 (2003).
Choi, H. J., Cohen, M. L. & Louie, S. G. Anisotropic Eliashberg theory of MgB2: Tc, isotope effects, superconducting energy gaps, quasiparticles, and specific heat. Physica C. 385, 66–74 (2003).
Pitt, M. P. et al. In situ neutron diffraction study of the deuteration of isotopic Mg11B2 . J. Phys. Chem. C. 115, 22669–22679 (2011).
Hishinuma, Y. et al. Microstructure and superconducting properties of Cu addition MgB2 multifilamentary wires using boron isotope powder as the boron source material. Phys. Procedia. 36, 1486–1491 (2012).
Lu, J. et al. Low-temperature synthesis of superconducting nanocrystalline MgB2 . J Nanomater. 2010, 191058 (2010).
Yamamoto, A. et al. Reactivity of carbides in synthesis of MgB2 bulks. Physica C. 445–448, 801–805 (2006).
Yamamoto, A. et al. Improved critical current properties observed in MgB2 bulks synthesized by low-temperature solid-state reaction. Supercond. Sci. technol. 18, 116–121 (2005).
Ma, Z. Q. & Liu, Y. C. The varied kinetics mechanisms in the synthesis of MgB2 from elemental powders by low-temperature sintering. Mater. Chem. Phys. 126, 114–117 (2011).
Ma, Z. Q., Liu, Y. C. & Gao, Z. M. The synthesis and grain connectivity of lamellar MgB2 grains by Cu-activated sintering at low temperature. Scripta Materialia. 63, 399–402 (2010).
Ma, Z. Q. et al. The mechanism of accelerated phase formation of MgB2 by Cu-doping during low-temperature sintering. Mater. Res. Bull. 44, 531–537 (2009).
Shi, Q. Z. et al. Phase formation process of bulk MgB2 analyzed by differential thermal analysis during sintering. J. Alloy. Compd. 458, 553–557 (2008).
Bean, C. P. Magnetization of hard superconductors. Phys. Rev. Lett. 8, 250–253 (1962).
Zhao, Q. et al. Characteristic and synthesis mechanism of MgB2 nanoparticles in solid-state reactive sintering. J. Alloy. Compd. 470, 443–447 (2009).
Ma, Z. Q. et al. The enhancement of Jc in nano SiC-doped MgB2 superconductors rapidly synthesized by activated sintering at low-temperature. Scripta. Mater. 61, 836–839 (2009).
Ma, Z. Q. et al. Effect of Cu addition in reduction of MgO content for the synthesis of MgB2 through sintering. J. Alloys Compd. 471, 105–108 (2009).
Yamamoto, A. et al. Improved critical current properties observed in MgB2 bulks synthesized by low-temperature solid-state reaction. Supercond. Sci. Technol. 18, 116–121 (2005).
Ye, S. J. et al. Enhancing the critical current properties of internal Mg diffusion-processed MgB2 wires by Mg addition. Supercond. Sci. Technol. 25, 125014 (2012).
Shi, Z. X. et al. Doping effect and flux pinning mechanism of nano-SiC additions in MgB2 strands. Supercond. Sci. Technol. 24, 065015 (2011).
Shi, Z. X. et al. Anisotropic connectivity and its influence on critical current densities, irreversibility fields, and flux creep in in situ processed MgB2 strands. Supercond. Sci. Technol. 23, 045018 (2010).
Ma, Z. Q. et al. MgB2 superconductors with abnormally- improved Jc sintered after autoxidation of milled original powders, J. Appl. Phys. 106, 113911 (2009).
Acknowledgements
This work is supported by the Australian Research Council (Grant No. DE140101333). The authors are grateful to China National Funds for Distinguished Young Scientists (Grant No. 51325401), the National Natural Science Foundation of China (Grant Nos 51302186 and 51574178), the National Magnetic Confinement Fusion Energy Research Project (grant No. 2014GB125006).
Author information
Authors and Affiliations
Contributions
Z.M. and Y.L. designed the experimental scheme. F.C. prepared the samples and did most of the characterizations. M.S.A.H. helped with the experiment and data analysis and M.S. provided the boron powder.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cheng, F., Liu, Y., Ma, Z. et al. Improved Superconducting properties in the Mg11B2 low activation superconductor prepared by low-temperature sintering. Sci Rep 6, 25498 (2016). https://doi.org/10.1038/srep25498
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25498
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.