Abstract
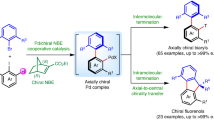
Fullerene chirality is an important but undeveloped issue of paramount interest in fields such as materials science and medicinal chemistry. So far, enantiopure fullerene derivatives have been made from chiral starting materials or obtained by separating racemic mixtures. Here, we report the enantioselective catalytic synthesis of chiral pyrrolidinofullerenes (the most widely studied fullerene derivatives), which proceeds in high yields under very mild conditions at low temperatures. The combination of a particular metal catalyst—Ag(I) or Cu(II)—and a chiral ligand is able to direct the cycloaddition of buckminsterfullerene C60, the first non-coordinating dipolarophile used in such reactions, to opposite enantiofaces of N-metallated azomethine ylides. This methodology has proven to be quite general, affording enantiomeric excesses of greater than 90%. Furthermore, well-defined chiral carbon atoms linked to the fullerene sphere are able to perturb the inherent symmetry of the fullerene π-system as revealed by circular dichroism measurements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
The Chemistry of Fullerenes (ed. Hirsch, A.) (Wiley-VCH, 2005).
Fullerenes: From Synthesis to Optoelectronic Properties (eds. Guldi, D. M., Martín, N.) (Kluwer Academic Publishers, 2002).
Martín, N. New challenges in fullerene chemistry. Chem. Commun. 2093–2104 (2006).
Thilgen, C. & Diederich, F. Structural aspects of fullerene chemistry: a journey through fullerene chirality. Chem. Rev. 106, 5049–5135 (2006).
Thilgen, C., Gosse, I. & Diederich, F. Chirality in fullerene chemistry. Top. Stereochem. 23, 1–124 (2003).
Friedman, S. H., Ganapathi, P. S., Rubin, Y. & Kenyon, G. L. Optimizing the binding of fullerene inhibitors of the HIV-1 protease through predicted increases in hydrophobic desolvation. J. Med. Chem. 41, 2424–2429 (1998).
Nishimura, T. et al. Macromolecular helicity induction on a poly(phenylacetylene) with C2-symmetric chiral [60]fullerene-bisadducts. J. Am. Chem. Soc. 126, 11711–11717 (2004).
Bianco, A. et al. Synthesis, chiroptical properties, and configurational assignment of fulleroproline derivatives and peptides. J. Am. Chem. Soc. 118, 4072–4080 (1996).
Illescas, B. et al. Diastereoselective synthesis of fulleropyrrolidines from suitably functionalized chiral cyclobutanes. J. Org. Chem. 70, 6929–6932 (2005).
Djojo, F. & Hirsch, A. Synthesis and chiroptical properties of enantiomerically pure bis- and trisadducts of C60 with an inherent chiral addition pattern. Chem. Eur. J. 4, 344–356 (1998).
Hawkins, J. M., Meyer, A. & Nambu, M. Asymmetric bisosmylation of C60: novel chiral π-systems. J. Am. Chem. Soc. 115, 9844–9845 (1993).
Vasella, A., Uhlmann, P., Waldraff, C. A. A., Diederich, F. & Thilgen, C. Fullerene sugars: preparation of enantiomerically pure, spiro-linked C-glycosides of C60 . Angew. Chem. Int. Ed. Engl. 31, 1388–1390 (1992).
Nájera, C. & Sansano, J. M. Catalytic enantioselective 1,3-dipolar cycloaddition reaction of azomethine ylides and alkenes: the direct strategy to prepare enantioenriched highly substituted proline derivatives. Angew. Chem. Int. Ed. 44, 6272–6276 (2005).
Pandey, G., Banerjee, P. & Gadre, S. R. Construction of enantiopure pyrrolidine ring system via asymmetric [3 + 2]-cycloaddition of azomethine ylides. Chem. Rev. 106, 4484–4517 (2006).
Gothelf, K. V. & Jørgensen, K. A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 98, 863–909 (1998).
Maggini, M., Scorrano, G. & Prato, M. Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 115, 9798–9799 (1993).
Martín, N. et al. Retro-cycloaddition reaction of pyrrolidinofullerenes. Angew. Chem. Int. Ed. 45, 110–114 (2006).
Pantarotto, D. et al. Solid-phase synthesis of fullerene-peptides. J. Am. Chem. Soc. 124, 12543–12549 (2002).
Longmire, J. M., Wang, B. & Zhang, X. Highly enantioselective Ag(I)-catalyzed [3 + 2] cycloaddition of azomethine ylides. J. Am. Chem. Soc. 124, 13400–13401 (2002).
Chen, C., Li, X. & Schreiber, S. L. Catalytic asymmetric [3 + 2] cycloaddition of azomethine ylides. Development of a versatile stepwise, three-component reaction for diversity-oriented synthesis. J. Am. Chem. Soc. 125, 10174–10175 (2003).
Cabrera, S., Gómez-Arrayás, R., Carretero, J. C. Highly enantioselective copper(I)-fesulphos-catalyzed 1,3-dipolar cycloaddition of azomethine ylides. J. Am. Chem. Soc. 127, 16394–16395 (2005).
Yan, X. X. et al. A highly enantio- and diastereoselective Cu-catalyzed 1,3-dipolar cycloaddition of azomethine ylides with nitroalkenes. Angew. Chem. Int. Ed. 45, 1979–1983 (2006).
Gothelf, A. S., Gothelf, K. V., Hazell, R. G. & Jørgensen, K. A. Catalytic asymmetric 1,3-dipolar cycloaddition reactions of azomethine ylides—a simple approach to optically active highly functionalized proline derivatives. Angew. Chem. Int. Ed. 41, 4236–4238 (2002).
Wang, C. J., Liang, G., Xue, Z. Y. & Gao, F. Highly enantioselective 1,3-dipolar cycloaddition of azomethine ylides catalyzed by copper(I)/TF-BiphamPhos complexes. J. Am. Chem. Soc. 130, 17250–17251 (2008).
Nájera, C., Retamosa, M. G. & Sansano, J. M. Catalytic enantioselective 1,3-dipolar cycloaddition reactions of azomethine ylides and alkenes by using Phosphoramidite–Silver(I) complexes. Angew. Chem. Int. Ed. 47, 6055–6058 (2008).
Wilson, S. R. et al. Chiral non-racemic C60 derivatives: a proposed sector rule for fullerene absolute configuration. Tetrahedron 52, 5131–5142 (1996).
Tan, X., Schuster D. I. & Wilson, S. R. Resolution and absolute configuration of a C2-symmetryc trans-2,5-disubstituted fulleropyrrolidine. Tetrahedron Lett. 39, 4187–4190 (1998).
Eliel, E. L. & Wilen, S. H. Stereochemistry of Organic Compounds, Ch 12 (Wiley, 1994).
Oderaotoshi, Y. et al. Exo- and enantioselective cycloaddition of azomethine ylides generated from N-alkylidene glycine esters using chiral phosphine-copper complexes. Org. Lett. 5, 5043–5046 (2003).
Vivanco, S. et al. Origins of the loss of concertedness in pericyclic reactions: theoretical prediction and direct observation of stepwise mechanism in [3 + 2] thermal cycloaddition. J. Am. Chem. Soc. 122, 6078–6092 (2000).
Houk, K., Gonzalez, J. & Li, Y. Pericyclic reaction transition states: passions and punctilios 1935–1995. Acc. Chem. Res. 28, 81–90 (1995).
Acknowledgements
This work was supported by the MICINN of Spain (project CT2008-00795/BQU and Consolider-Ingenio 2010C-07-25200) and the CAM (project P-PPQ-000225-0505). S.F. thanks the MICINN for a Ramón y Cajal contract, and E.E.M. thanks the MICINN for a Doctoral Fellowship.
Author information
Authors and Affiliations
Contributions
N.M. and S.F. conceived and designed the experiments; E.M. and M.S. performed the experiments; A.M. analysed the data; N.M. and S.F. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 3669 kb)
Rights and permissions
About this article
Cite this article
Filippone, S., Maroto, E., Martín-Domenech, Á. et al. An efficient approach to chiral fullerene derivatives by catalytic enantioselective 1,3-dipolar cycloadditions. Nature Chem 1, 578–582 (2009). https://doi.org/10.1038/nchem.361
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.361
This article is cited by
-
Steering the asymmetric folding of a nanographene
Nature Synthesis (2024)
-
Non-spherical gold nanoparticles enhanced fluorescence of carbon dots for norovirus-like particles detection
Journal of Biological Engineering (2023)
-
Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
Nature Chemistry (2023)
-
Copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of 1,3-enynes and azomethine ylides
Nature Communications (2023)