« Prev Next »

Defining and Measuring Communities
No species occurs in isolation. All living organisms live and interact with other species and are integral members of an ecological community: an assemblage of sympatric, synchronic species. Both long-term (evolutionary time scale) and short-term (ecological time scale) processes influence the composition of species within a community and the interactions among them. Longer-term evolutionary and biogeographic processes shape the features relevant to species interactions and patterns of species distribution, richness and abundance, while short term ecological interactions can influence the intensity of an interaction and the presence and abundance of species over the course of a season, a year, or within the lifetime of an organism (Morin 1999, Verhoef & Morin 2010).
Subsets of species in a community: Taxocenes & guilds
Food webs in a community
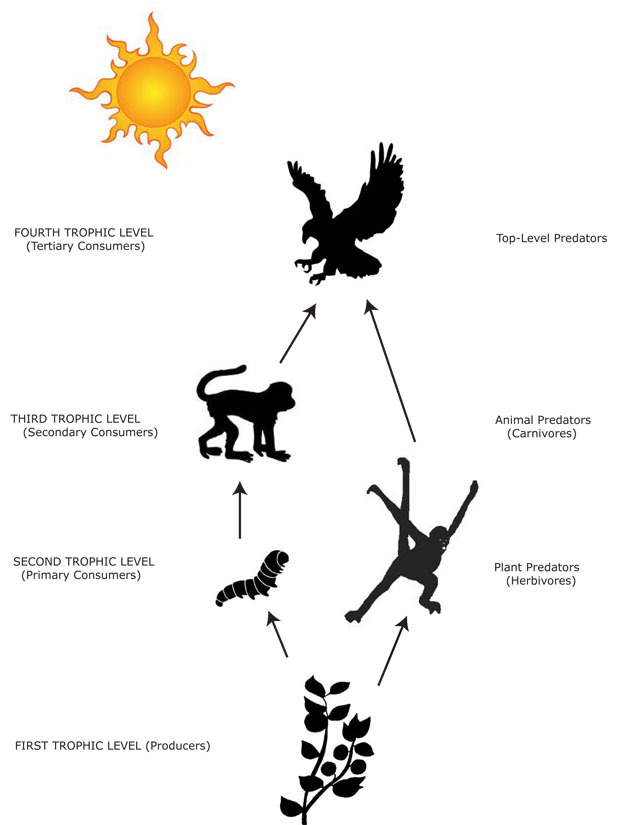
The position a species occupies in a food web is a central determinant of its interactions with other species. Essentially, food webs are the sum of all predator-prey interactions at work in an assemblage, and are a depiction of how solar-derived energy moves among species. An organism's position in this web is described as its trophic level (Figure 1). The first trophic level comprises plants (autotrophs) that utilize solar energy by converting carbon dioxide into organic compounds, especially sugars, in a process known as photosynthesis. Plants are thus called producers because they are the basis upon which heterotrophic organisms exist — organisms known as consumers. Primary consumers occupy the second trophic level and are plant-consuming animals — herbivores. Secondary consumers represent the third trophic level, consume herbivores, and are called carnivores. Carnivores can also consume carnivores and occupy the forth trophic level, and so on. Animals that eat food from more than one trophic level are called omnivores. Most primates are omnivores, although there are several groups of primates that have adaptations for pure herbivory (e.g., Colobinae, Alouatta spp.) or carnivory (e.g., Tarsius spp.).
Species interactions in a community
Because species are inextricably linked to each other in food webs, communities are best understood as an assemblage of species interactions of who is eating whom. These interactions will have either a negative (–), positive (+), or neutral (0) consequence for each of the two (or more) species, and thereby have extremely important consequences for the ecology, adaptations, and evolutionary history of all organisms. Since at least two species are involved, an interaction can be defined in terms of the plusses and minuses in the interaction to those two species (Table 1). For example, predation is an interaction in which one species benefits (+) because it has procured its dinner, while the other species is clearly negatively (–) impacted because it is the dinner (Figure 2).
| Interaction |
Species One
|
Species Two
|
|
Predation |
– | + |
| Parasitism | – | + |
| Competition | – | – |
| Mutualism | + | + |
| Ammensalism | 0 | – |
| Commensalism | 0 | + |
| Table 1. Species interactions defined in terms of the negative (–), positive (+), or neutral (0) impact of the interaction on each of the two species. | ||

Primates in communities
Competitive Interactions
Within a community, all species — including primates — occupy their own, unique niche. Although it is a difficult concept to define, a niche can be generally understood as the position of an organism in a community and the ecological roles it plays in that community. Hutchinson (1957) provided a more explicit definition that identifies a niche in n-dimensional hypervolume in which the dimensions are the environmental conditions that define the range in which a species can persist. The fundamental niche is the full range of environmental conditions in which a species can exist; a realized niche is a narrower set of environmental conditions that a species exploits in the presence of competition. The potential for competition (scramble or contest) is greatest among closely related, sympatric species that occupy similar niche space. Interspecific competition is also often most intense when resources are either temporally or spatially scarce and/or highly favored as a function of their quality. When extant species are observed or expected to compete directly for critical resources, one or more of the interacting species are predicted to undergo population decline or character displacement in a process known as competitive exclusion (Gause 1934).
That primates compete with one another intraspecifically is well demonstrated and has received considerable research attention, particularly with regards to male-male competition, female-female competition, and inter-group competition (Strier 2011). These are population level interactions, however. Interspecific competition is much more difficult to demonstrate, especially with long-lived species such as primates. In short, to demonstrate that one species is out-competing another species, it must be shown that the presence of the superior competitor is negatively impacting the abundance or microevolution of the other species. Suggesting that species' adaptations and local abundance are a function of past interspecific competition makes several assumptions. First, it assumes that the environment is constant over time, and that the variables shaping interactions in the present are the same variables that were important in the ecological and evolutionary past ("Ghost of Competition Past", see Connell 1980). It also assumes that population growth in species a is determined by species b, when in fact there are many non-competition related factors that influence population abundance (e.g., predation, resource availability, disease). No one doubts that interspecific competition over resources can be important in shaping community interactions and assemblages. However, the difficulty of demonstrating this empirically has led many researchers to evaluate species coexistence, which rather than theorizing past competition instead focuses on the processes and mechanisms allowing extant species to live sympatrically in the ecological present (Lambert 2002, Tokeshi 1999).
Predator-Prey Interactions
Primates as predators
Primates can be both predator and prey species. As predators, primates prey on plants (herbivory) and animals (carnivory). Herbivory evolved approximately 255 million years ago and was a dramatic shift from previous periods that lacked primary consumers (Potts 1996). Throughout the Mesozoic Era, angiosperms diversified; in turn, mammals and other organisms radiated into a wide range of plant-consuming niches. Primates are thus among these modern mammal orders that evolved to feed primarily on plants, such that even omnivorous primates eat plants to meet a majority of their nutritional and energetic requirements. Some primates such as howler monkeys (Alouatta spp.), mountain gorillas (Gorilla gorilla berengei), leaf-eating monkeys (colobinae), and saki monkeys (pitheciinae) have a diet composed entirely of leaves or seeds and other plant parts (Figure 3). These species have evolved features of the dentition and gut that facilitate their obligate folivory (leaf eating) or granivory (seed eating) (Lambert 1998). Pitheciine monkeys are specialized seed predators and have dental anatomy specialized for cracking open hard, unripe fruit to exploit immature seeds; in this role, pitheciines are part of a seed predator guild that includes parrots (Norconk et al. 1998). Colobine monkeys have evolved specialized stomachs that ferment plant fiber and facilitate a plant diet, similar to ruminant ungulates (Lambert 1998). Folivorous primates have the potential to strip trees of their leaves and kill them; for example, Glander (1975) documented tree mortality from the constant feeding pressure of an Alouatta palliata group in Costa Rica. Even if they do not kill the tree completely, folivorous primates can exert considerable leaf predation pressure, particularly in communities in which leaf-eating monkeys are highly abundant. In Kibale National Park for example, Struhsaker (1997) documents an average of 356 individual colobines living in a square kilometer, each of which spends most of its day consuming leaves and other plant parts.

Most animals preyed on by primates are invertebrates — particularly insects — and small vertebrates including reptiles, amphibians, birds, and bird eggs. Some larger-bodied primates (e.g., Papio anubis, Pan troglodytes) consume larger vertebrates such as forest duiker, impala, bushbuck, bush pigs, and even other primates such as galagos (Galago spp.). However, it is the chimpanzee (Pan troglodytes) that has received most attention as a predator species. While other primates tend to be categorized as opportunistic predators, chimpanzees appear to be highly organized and hunt cooperatively (Figure 2). Although chimpanzees consume a variety of animal prey, it is their consumption of another primate species — red colobus (Procolobus badius) — that is most conspicuous. Wherever red colobus are sympatric with chimpanzees, the former is hunted and consumed by the latter. This can have a serious impact on the abundance of red colobus; at Ngogo, Uganda, chimpanzees consume an average of 3% of red colobus population per annum (Mitani & Watts 1999). In Gombe National Park, Tanzania, it has been estimated that in the absence of chimpanzee predation, red colobus populations would increase by 100% in ten years (Stanford 1998).
Primates as prey
Parasite-Host Interactions
Similar to predator-prey interactions, parasite-host interactions clearly have negative consequences for one species, and a positive outcome for the other (– , +). Primates can serve as hosts to many parasites. Some parasite-host interactions may only have a mild impact upon the health of the host, while others can impact fitness and longevity of the host. Ectoparasites are external parasites that can be found in the fur, skin, and superficial flesh of their hosts. A common primate ectoparasite is the neotropical botfly, which lay eggs in flesh for the developing larvae to feed on. Left undisturbed, they develop into large flies; scratching can lead to skin infections that may be responsible for high mortality among howler monkeys (Milton 1996). Endoparasites are parasites found internally to the host, most commonly in the gastrointestinal tract, but also in blood, lungs, and other body tissues and organs. Gastrointestinal parasites in particular are quite common and typically more deleterious to the host than are ectoparasites; these parasites are typically transmitted by eating soiled vegetation or consuming insects and other animals that contain the parasites in their body tissues.
A host's parasite load can vary by individual, season, and region. For example, in both the neo- and paleo-tropics, there is a higher prevalence of parasitic infections and also more opportunities for re-infection during wetter seasons. When parasites are deleterious to host, they are a potentially powerful selective force on primates and can favor behaviors that reduce risk of becoming infected (Stuart & Strier 1995). For example, it is known in some species (e.g., Alouatta palliata) that animals will more often switch sleeping sites during seasons of high parasite abundance (Milton 1996). In addition, social groups will avoid certain travel routes have lower incidences of parasites than groups that re-use sleeping trees and travel routes.
Primate Mutualisms
Polyspecific Associations
Seed dispersal
Primates can also be important mutualists with plants. By far the most common mutualistic primate-plant interaction occurring in communities is seed dispersal (Figure 4). Parent plants rely on frugivorous animals such as primates to move their seeds, because it is in the best interest of a plant's fitness for seeds to escape the typically high density-dependent mortality common beneath a parent tree's crown. Both seed predators (e.g., bruchid beetles, rodents) and seed pathogens (e.g., bacterial, fungal) are attracted to the tree crown area and thus those seeds that are removed and carried some distance away are more likely to survive and germinate. In exchange for this ecological service (sensu Howe 1988) frugivores earn their ecological reward in the form of their food (sugar-rich fruit pulp).

Because of the extreme selection upon plant fitness, plant species around the world have evolved a number of mechanisms to disperse their seeds including a number of abiotic mechanisms such as wind and water. In the tropics, plants are more likely to rely on animals (zoochory). Because fruit can — in theory — be available year-round in less seasonal habitats at lower latitudes, most frugivorous animal species are found in the tropics. Plants have thus evolved a number of ways to attract frugivores and provide them with an ecological reward in the form of fruit pulp that is typically high in simple sugars. Animals in the tropics also exhibit a diversity of adaptations for frugivory, and many primates have a diet that has a high percentage of fruit.
As many as 75% of tropical tree species produce fruits that exhibit adaptations for zoochory, and animals have been proposed to disperse the seeds of 95% of all tropical seeds. Although many animal species disperse seeds (e.g., ants, beetles, fish, reptiles, rodents, elephants, bats, birds), primates are particularly important because they very often highly frugivorous and also because they are large-bodied and abundant. In Colombia, woolly monkeys (Lagothrix lagothricha) can disperse 25,000 seeds/km2/day (Stevenson 2000). In Costa Rica, spider monkeys (Atleles geoffroyi) and capuchins (Cebus capucinus) can disperse upwards of 5,600 seeds/km2/day (Chapman 1989). And, in Uganda, two monkey species (Cercopithecus ascanius and C. mitis) can move 33,840 seeds/km2/day, and chimpanzees can move seeds (via swallowing and defecation) of 1,398 seeds/km2/day (Lambert 2011). Because these seeds have been moved away from the parent tree crown environment, their likelihood of surviving and germinating is greatly enhanced.
Glossary
Allopatry: Not occurring in the same place or time.
Angiosperms: Flowering plants.
Autotrophic: An organism that is able to form nutritional organic substances from simple inorganic substances such as carbon dioxide.
Biogeography: The study of the spatial and temporal distribution of species.
Carnivores: Animals that consumer other animals.
Character displacement: A phenomenon in which differences between similar species with overlapping geographic distributions are greater in sympatry but minimized or lost in allopatry.
Community: An assemblage of sympatric (same area), synchronic (same time) species.
Competitive exclusion: States that two species competing for the same resources cannot coexist if other ecological factors are constant.
Contest competition: A form of competition where there is a winner and a loser and where resources can be completely attained or not at all.
Ectoparasites: External parasites that can be found in the fur, skin, and superficial flesh of their hosts.
Endoparasites: Internal parasites found typically the gut or blood.
Folivory: Leaf eating.
Frugivory: Fruit eating.
Fundamental niche: The full range of environmental conditions in which a species can exist.
Granivory: Seed eating.
Guild: A subset of species within a community that use a set of resources in similar ways.
Heterotrophic: An organism that cannot synthesize its own food and is dependent on complex organic substances for nutrition.
Inter: Between.
Intra: Within.
Parasite: An organism that benefits at the expense of another organism (host).
Photosynthesis: The synthesis of complex organic materials, especially carbohydrates, from carbon dioxide, water, and inorganic salts, using sunlight as the source of energy and with the aid of chlorophyll and associated pigments.
Population ecology: The study of the vital statistics of individuals of the same species, and the interactions among them.
Polyspecific associations: Associations in which sympatric social groups of two or more species aggregate.
Producers: Producers are responsible for the production of organic compounds from atmospheric or aquatic carbon dioxide. They form the base of the food chain.
Realized niche: The subset of environmental conditions that a species exploits in the presence of competing species.
Ruminant ungulates: Hooved mammals with specialized, sacculated stomachs that house microbes capable of fermenting cellulose and other components of plant fiber.
Scramble competition: A finite resource that is shared equally amongst the competitors so that the quantity of food per individual declines with increasing population abundance.
Species abundance: Number of individuals of a species in an area.
Species richness: Number of species in an area.
Sympatric: Species occupying the same place.
Synchronic: Species existing at the same time.
Taxocene: A subset of species within a community sharing a common taxonomic classification.
Zoochory: Seed dispersal by animals.
References and Recommended Reading
Chapman, C. A. Primate seed dispersal: The fate of dispersed seeds. Biotropica 21, 148-154 (1989).
Clements, F. E. Plant Succession. Washington, DC: Carnegie Institute of Washington, 1916.
Connell, J. H. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35, 131-13 (1980).
Fleagle, J. G. et al. Primate Communities. Cambridge, UK: Cambridge University Press, 1999.
Gause, G. F. The struggle for existence. Baltimore, MD: Williams & Wilkins, 1934.
Glander, K. H. "Habitat description and resource utilization: A preliminary report on mantled howler monkey ecology," in Socioecology and Psychology of Primates, ed. R. H. Tuttle (The Hague, Netherlands: Mouton, 1975), pp 37 - 57.
Howe, H. F. Ecological Relationships of Plants and Animals. Oxford, UK: Oxford University Press, 1988.
Hutchinson, G. E. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22, 415-427 (1957).
Isbell, L. A. Predation on primates: Ecological patterns and evolution consequences. Evolutionary Anthropology 3, 61-71 (1994).
Isbell, L. A. & Young T. P. Human presence reduces predation in a free-ranging vervet monkey population in Kenya. Animal Behaviour 45, 1233-1235 (1993).
Jaffe, K .E. & Isbell, L. "The Guenons: Polyspecific associations in sociological perspective," in Primates in Perspective, eds. C. J. Campbell et al. (Oxford, UK: Oxford University Press, 2010), pp 277 - 300.
Lambert, J. E. Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evolutionary Anthropology 7, 8-20 (1998).
Lambert, J. E. "Resource switching in guenons: A community analysis of dietary flexibility," in The Guenons: Diversity and Adaptation in African Monkeys, eds. M. Glenn & M. Cords (New York, NY: Kluwer Academic Press, 2002), pp 303-317.
Lambert, J. E. Primate seed dispersers as umbrella species: A case study from Kibale National Park, Uganda, with implications for Afrotropical forest conservation. American Journal of Primatology 73, 9-24 (2011).
Milton, K. Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. Journal of Zoology 239, 39-63 (1996).
Mitani, J. C. & Watts. D. Demographic influences on the hunting behavior of chimpanzees. American Journal of Physical Anthropology 109, 439-454 (1999).
Mitani, J. C. et al. Predatory behavior of crowned hawk-eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behavioral Ecology and Sociobiology 49, 187-195 (2001).
Morin, P. J. Community Ecology. Oxford, UK: Blackwell Science, 1999.
Norconk, M. A. et al. Seed dispersal by neotropical seed predators. American Journal of Primatology 45, 103-126 (1998).
Potts, R. Humanity's Descent: The Consequences of Ecological Instability. New York, NY: William Morrow & Company, 1996.
Struhsaker, T. T. Polyspecific associations among tropical rain forest primates. Zietschrift fur Teirpsychologie 57, 268-304 (1981).
Struhsaker, T. T. Ecology of an African Rain Forest: Logging in Kibale and the Conflict Between Conservation and Exploitation. Gainesville, FL: University Press of Florida, 1997.
Strier, K. B. Primate Behavioral Ecology, 4th ed. New York, NY: Prentice Hall, 2011.
Stanford, C. B. Chimpanzee and Red Colobus: The Ecology of Predator and Prey. Cambridge, MA: Harvard University Press, 1998.
Stevenson, P. R. Seeds dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Columbia: Dispersal distance, germination rates and dispersal quality. American Journal of Primatology 50, 275-289 (2000).
Stuart, M. D. & Strier, K. B. Primates and parasites. International Journal of Primatology 16, 577-593 (1995).
Tokeshi, M. Species Coexistence: Ecological and Evolutionary Perspectives. Oxford, UK: Blackwell Science, 1999.
Verhoef, H. A. & Morin, P. J. Community Ecology: Processes, Models, and Applications. Oxford, UK: Oxford University Press, 2010.