« Prev Next »
 Despite the wonders cell culturing has given us in terms of basic research and potential cell-based therapies (and even art), many of the principles and methods have remained relatively unchanged for the past 50 years, and with them, their many limitations. Typical cell culture is a lot like nurturing a plant, we seed cells onto a plastic dish filled with liquid nutrients, let them settle and stick to the dish, and then periodically change the media until the cells start to outgrow their confines. At which point, we tear them off the dish using enzymes and a bit of gentle force, then re-seed a small fraction of the cells onto a new dish.*
Despite the wonders cell culturing has given us in terms of basic research and potential cell-based therapies (and even art), many of the principles and methods have remained relatively unchanged for the past 50 years, and with them, their many limitations. Typical cell culture is a lot like nurturing a plant, we seed cells onto a plastic dish filled with liquid nutrients, let them settle and stick to the dish, and then periodically change the media until the cells start to outgrow their confines. At which point, we tear them off the dish using enzymes and a bit of gentle force, then re-seed a small fraction of the cells onto a new dish.*
There's no denying the fact that cultured cells are growing in a completely artificial and foreign environment. This strangeness prompts the cells to transform and to adapt. This transformation is an understated, but significant, caveat of cell culture and it's the reason why cell cultures do not faithfully reflect native cell function or physiology. Stem cell biologists are keenly aware of these challenges because the main goal of stem cell studies and regenerative medicine is to produce functional tissues outside the body. The obvious answer to these problems is to recapitulate synthetically as many of the body's physiological conditions as possible. It is equally obvious that cell culture methods must outgrow flat, 2D plastic dishes.
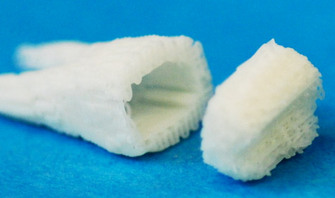
The past few years have seen the rise of bioengineering, also called tissue engineering, which uses insights from cellular biology to help design synthetic materials and 3D culture systems that mimic a cell's "natural" surroundings. The ideal environment for most cells would be some form of stationary scaffold (analogous to extracellular matrix) exposed to circulating cytokines, metabolites, and hormones (analogous to circulating blood vessels). Bioengineers must also work within other practical constrains, such as biocompatibility and biodegradability, when designing synthetic materials. So far, biomimic materials such as nanofibers, hydrogels, biofoams, and other porous materials have been made using biodegradable polymers that are intended for tissue repairs and to replace the typical culture dish.1 The advantages of these materials are that they can be engineered to very precisely mimic the thickness, stiffness, or permeability of natural matrices.1 They are being incorporated into new bioreactor designs that circulate nutrients and metabolites through the synthetic matrix, much like the way blood vessels feed tissues, and the resulting grafts and organs are transplantable. It was just over a year ago when a lab-grown trachea became the first synthetic organ to be transplanted into a patient in Stockholm.
Scaffold-bioreactor systems have been able to synthesize a variety of other tissues, including bone, skin, blood vessels, and muscles.1,2 Such complementary culture systems have been especially useful for growing muscles or bones because their precursor cells need mechanical or electrical stimulation to fully mature into functional tissues. Under standard culture conditions, the precursor cells typically remain immature, do not self-organize into tissues, and are non-functional. For example, researchers have placed flexible matrices impregnated with immature muscle cells inside advanced bioreactors equipped with a mechanical force generator, which basically pull or twist the matrix at a set frequency to exercise the immature cells, and found that they self-organize and assemble into more complex structures similar to functional muscles.1,2 Fewer studies have been done to study the effects of electrical fields, but preliminary studies also suggest the cells will align themselves based on polarity and form higher-order structures.1,2
So, will organ donations be obsolete? Not anytime soon. Bioengineering still rely on information from the biological sciences to guide designs, and for many organs, we still don't have the complete recipe and don't know what we need to fully mimic native environments. However, it is quite probable that developmental biology combined with high-throughput technologies1,2 will fill in many of these knowledge gaps and become highly influential in the future.
*As an aside, we re-seed cells because the cells will starve to death and die if overgrown; not because they may spill over the dish, grow into a sentient blob, and take over the world.
Photo credit: Columbia University via LabGrab.com (http://bit.ly/MnCBVP)
References:
- Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011 Mar 4;8(3):252-61.
- Grayson WL, Martens TP, Eng GM, Radisic M, Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009 Aug;20(6):665-73. Epub 2008 Dec 25.























http://www.fluoresentric.com