Abstract
Background:
Abnormal PCO2 is common in infants with hypoxic ischemic encephalopathy (HIE). The objective was to determine whether hypocapnia was independently associated with unfavorable outcome (death or severe neurodevelopmental disability at 18 mo) in infants with moderate-to-severe HIE.
Methods:
This was a post hoc analysis of the CoolCap Study in which infants were randomized to head cooling or standard care. Blood gases were measured at prespecified times after randomization. PCO2 and follow-up data were available for 196 of 234 infants. Analyses were performed to investigate the relationship between hypocapnia in the first 72 h after randomization and unfavorable outcome.
Results:
After adjusting for pH, amplitude-integrated electroencephalogram background and seizures, birth weight, Apgar score at 5 min, cooling status, and Sarnat stage, PCO2 was inversely associated with unfavorable outcome (P < 0.001). The probability of unfavorable outcome was 0.20 ± 0.1 (point estimate ± SE), 0.53 ± 0.23 and 0.89 ± 0.16 for a PCO2 of 40, 30, and 20 mm Hg respectively and was greater in infants with severe HIE than with moderate HIE.
Conclusions:
Hypocapnia in infants with moderate-to-severe HIE was independently associated with unfavorable outcome. Future studies of controlled normocapnia will be important.
Similar content being viewed by others
Main
The cerebral vasculature is exquisitely sensitive to changes in the partial pressure of carbon dioxide (PCO2). Hypercapnia leads to cerebral vasodilation and hyperperfusion and, reciprocally, hypocapnia is a potent mediator of cerebral vasoconstriction leading to decreased cerebral blood flow (1). Hypocapnia has been associated with brain injury in animals and human infants (2,3,4,5,6,7,8,9,10,11). In a large retrospective cohort study of infants with hypoxic ischemic encephalopathy (HIE), severe hypocapnia was associated with increased risk of subsequent death or severe neurodevelopmental impairment, with an odds ratio of 2.3 (12). Consistent with this, in the National Institute of Child Health and Human Development Neonatal Research Network randomized, controlled trial of whole-body hypothermia in term infants with HIE, both minimum and cumulative exposure to hypocapnia in the first 12 h of the trial were associated with death or adverse neurodevelopmental outcome (13). The dose–response relationship between PCO2 and outcome for infants with moderate-to-severe HIE is unknown.
The risk of developing hypocapnia or hypercapnia may be affected by severity of brain injury, intensity and duration of newborn resuscitation, timing after the primary injury, and/or response to metabolic acidosis. Thus, in this secondary analysis of the CoolCap Study (14), we sought to confirm the observation that hypocapnia in infants with moderate-to-severe HIE is associated with unfavorable outcome at 18 mo. Further, we tested the hypothesis that unfavorable outcome would be dose-dependently associated with decreasing PCO2 and that hypocapnia would be associated with a greater adverse effect in infants with severe than in those with moderate HIE.
Results
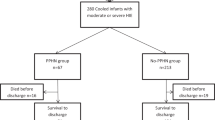
Out of the 234 infants enrolled in the original CoolCap study, 8 had mild HIE and another 5 did not have Sarnat stage recorded at randomization, leaving 221 infants with moderate-to-severe HIE. Complete follow-up data for infants with moderate-to-severe HIE was available for 206 infants, and an additional 10 infants had missing demographic and neonatal variables, leaving 196 infants for the current study ( Figure 1 ).
CONSORT chart showing flow of infants in the study.
The mean (SD) birth weight and gestational age of the study cohort was 3,451 (640) g and 39.1 (1.5) weeks. Ninety-eight (50%) infants received therapeutic hypothermia. Overall, 63.3% (124 of 196) of infants had moderate HIE and 36.7% (72 of 196) had severe HIE at randomization. Blood gas data was available for 192 infants at randomization, 189 at 4 h, 188 at 8 h, 185 at 12 h, 175 at 24 h, and 156 infants at 72 h. All infants were mechanically ventilated at randomization. The mean (SD) PCO2 in the first 72 h was 36.2 (10.2) mm Hg (range of 6–117.6 mm Hg). Hypocapnia (PCO2 less than 30 mm Hg) at randomization was present in 40.0 % (48 of 120) of infants with moderate and 66.7% (48 of 72) infants with severe HIE. In addition, 63.3% (124 of 196) of infants survived, and 62.2% (122 of 196) had unfavorable outcome ( Table 1 ).
Unfavorable outcome was associated with higher birth weight, lower Apgar scores at 5 min, severe HIE, and worse neurologic status (abnormal amplitude integrated electroencephalogram background and/or seizures) at randomization ( Table 1 ).
In a generalized additive model, there was a significant nonlinear association between PCO2 and unfavorable outcome (P < 0.001). In addition, Apgar score at 5 min less than 5 (vs. greater than or equal to 5), Sarnat stage 3 (vs. stage 2), larger birth weight, and standard therapy (vs. head cooling) were also independently associated with increased risk of unfavorable outcome in this model ( Table 2 ).
PCO2 increased over time and was significantly higher at 12 (P < 0.01), 24 (P < 0.001), and 72 h (P < 0.001) than at initial randomization ( Figure 2a ). Sarnat stage at randomization did not have a main effect on the PCO2 trend whereas cooled infants had higher PCO2 than infants in the control group (two-way ANOVA, P = 0.002, Figure 2b ).
Temporal PCO2 pattern during treatment. (a) Temporal pattern of PCO2 between infants with moderate (solid line) or severe hypoxic ischemic encephalopathy (HIE) (dashed line). Significant differences in PCO2 compared to levels at randomization are represented by **P < 0.01. (b) Temporal pattern of PCO2 between infants with HIE who underwent head cooling (dashed line) compared to those managed with standard care (solid line). Significant differences in PCO2 (cooled vs. control groups) are represented by *P < 0.05. Values represented are mean and SD at each time point.
The probability of an unfavorable outcome was significantly increased at lower PCO2 values: 0.20 ± 0.1 (point estimate ± SE) for a PCO2 of 40 mm Hg, 0.53 ± 0.23 for a PCO2 of 30 mm Hg, 0.74 ± 0.23 for a PCO2 of 25 mm Hg, 0.89 ± 0.16 for a PCO2 of 20 mm Hg, and 0.96 ± 0.08 for a PCO2 of 15 mm Hg. For PCO2 values from 20 to 40 mm Hg, hypocapnia was associated with a higher probability of unfavorable outcome in infants with severe compared to those with moderate HIE ( Figure 3 ).
Effect of PCO2 on the adjusted probability of an unfavorable outcome for infants with moderate (blue curve) and severe hypoxic ischemic encephalopathy (red curve) at randomization. The shaded areas around the curves represent the standard error of mean of the point estimate of the probability of unfavorable outcome at a given PCO2. The darkly shaded area indicates the overlap in the distribution.
Discussion
In this secondary analysis of infants with moderate-to-severe HIE from the CoolCap Study, we confirmed that hypocapnia was associated with increased risk of unfavorable outcome (death or severe neurodevelopmental disability) at 18 mo of age. Further, we observed that the probability of an unfavorable outcome increased dose-dependently with decreasing PCO2, and that hypocapnia was associated with a higher probability of unfavorable outcome in infants with severe compared to those with moderate HIE.
The present findings are consistent with previous reports. In a retrospective cohort study of term infants with early-onset HIE, severe hypocapnia (PaCO2 less than 20 mm Hg) during the first 20–120 min of life was associated with death or severe neurodevelopmental disability (odds ratio 2.34, 95% CI: 1.02–5.37, P = 0.044) (12). Similarly, Pappas et al. (13) found that cumulative exposure to PCO2 less than 35 mm Hg (the difference between 35 mm Hg and the sampled PCO2 multiplied by time below the threshold) from birth to 12 h of life in infants with moderate-to-severe HIE was associated with death or moderate/severe neurodevelopmental disability at 18–22 mo. The present study now suggests for the first time the possibility that hypocapnia may be particularly detrimental in infants with severe HIE.
It is not possible to determine from these exploratory data whether the association between hypocapnia and death or disability is causal. All of the infants in our study received mechanical ventilation at randomization. Thus, the frequent hypocapnia may reflect overzealous newborn resuscitation and mechanical ventilation. Alternatively, hypocapnia may be a proxy for the severity of brain injury, since severely damaged tissues show reduced net glucose metabolism, and so generate less CO2 (15). Further, it is possible that greater hypotonia associated with more severe HIE could facilitate overventilation (16). Nevertheless, in the present study, the association of unfavorable outcome with hypocapnia remained high despite statistical adjustment for severity of HIE, pH, and other variables. Further, HIE does not impair hypocapnia-mediated cerebral vasoconstriction (17), and the effects of hypocapnia on cerebral vascular tone persist during therapeutic hypothermia (18). Thus, infants with HIE undergoing hypothermia are still vulnerable to the potentially harmful effects of hypocapnia, and so it is plausible hypocapnia may be a modifiable risk factor for improving neurological outcomes.
This possibility is supported by observations in animal models of cerebral hypoxia-ischemia. Hypocapnia from overventilation decreased oxidative metabolism and increased DNA degradation in the cerebral cortex of normoxic newborn piglets (2). Normocapnia protected the immature rat brain compared to hypocapnia after hypoxia–ischemia; mild hypercapnia was even more protective than normocapnia (19). Cerebral high-energy phosphate reserves were preserved in normo- and hypercapnic animals compared to those exposed to hypocapnia (20). Similarly, Zhou et al. (21) reported that in adult male rats exposed to transient global cerebral ischemia-reperfusion injury, mild-to-moderate hypercapnia improved neurobehavioral recovery and reduced histological injury compared to normocapnia or severe hypercapnia. Consistent with this, in adult rats after focal cerebral ischemia/reperfusion, hypercapnia decreased neuronal apoptosis and improved spatial memory and sensorimotor impairment (22).
Hypothermia is known to decrease PCO2 due to decreased CO2 production because of decreased cerebral metabolism (23) and increasing solubility of CO2 in blood (24), and therefore could predispose cooled infants to hypocapnia. In the present study, however, cooled infants had significantly higher PCO2 than infants receiving standard care at 8, 24, and 72 h. Potentially, such an increase in PCO2 could modulate the neuroprotective effects of mild hypothermia and help preserve cerebral aerobic metabolism compared to noncooled infants with HIE. Speculatively, such mildly increased PCO2 in the cooled infants in turn could have an additional neuroprotective effect, given the evidence that mild hypercapnia is protective in animal models (19,21).
Some limitations of the present study should be taken into consideration. The analysis was post hoc; however the data were collected prospectively. We do not have PCO2 values between the prespecified time points and therefore it is possible that we underestimated the true incidence and depth and duration of hypocapnia. Further, without continuous monitoring of PCO2, fluctuations in PCO2 that are common in infants on mechanical ventilation were likely to have been missed. However, the prespecified timing of PCO2 levels that were measured independently of clinical decisions strengthens the study. Continuous real-time monitoring using transcutaneous PCO2 may have given better insights into the effect of changes in the ventilator settings. However, peripheral vasoconstriction associated with hypothermia may make transcutaneous blood gas monitoring problematic. It is also important to note that the probability of unfavorable outcome, as represented in Figure 3 , as 100% at PCO2 of 20 mm Hg and 0% at PCO2 of 60 mm Hg as generated by the statistical model, should be interpreted with caution as the number of values at these extremes was very small. Also, in the CoolCap Study, the effect of PCO2 on cerebral blood flow was not monitored, and data on clinical ventilator management in response to blood gases were not collected.
The present study shows that hypocapnia was common in infants with moderate-to-severe HIE, and associated with unfavorable outcome at 18 mo of age. There was a dose-dependent risk of poor outcome with hypocapnia, such that infants with lower PCO2 had a higher probability of unfavorable outcome. Moreover, the probability of unfavorable outcome with hypocapnia was greater in infants with severe HIE. Hypocapnia may simply be a biomarker of brain injury. However, given the preclinical evidence that hypocapnia contributes to ongoing injury after hypoxic–ischemic injury, and the consistent association in large clinical studies, formal trials of controlled normocapnia in infants with moderate-to-severe HIE are now essential. Until such trials are completed, pragmatically it is reasonable for clinicians to avoid hyperventilation in infants with moderate-to-severe HIE and to aim to facilitate early extubation in spontaneously breathing infants on mechanical ventilation.
Methods
Subjects
The CoolCap Study was a multicenter randomized controlled study of selective head cooling and mild systemic hypothermia for the treatment of perinatal moderate-to-severe HIE in 234 infants greater than or equal to 36 weeks gestation enrolled from 1999 to 2002 (14). This study was performed in 25 perinatal centers using a trial design registered with the US Food and Drug Administration under the Investigational Device Exemption/Premarket Approval program. The following institutions participated in this study: University of Auckland-National Women’s Hospital, NZ, Southmead Hospital. Bristol, UK, St. Michael’s Hospital, Bristol, UK, University College Hospital, London, UK, Hammersmith Hospital, London, UK, Royal Alexandra Hospital/University of Alberta Hospital, CA, Arkansas Children’s Hospital, Children’s Memorial Hospital and Prentice Women’s Hospital of Northwestern Memorial Hospital, University of Illinois at Chicago, Children’s Hospital of New York-Presbyterian, Columbia University, Children’s Hospital of Denver, Duke University, Johns Hopkins University, University of Michigan-Mott Children’s Hospital, Children’s Hospital and Clinics of Minneapolis, Children’s Hospital and Research Center at Oakland, Children’s Hospital of Oklahoma, Children’s Hospital of Eastern Ontario/The Ottawa Hospital, AI Dupont Children’s Hospital at Thomas Jefferson University, Magee Women’s Hospital/ Children’s Hospital of Pittsburgh, Golisano Children’s Hospital at Strong, University of California San Diego Medical Center (Hillcrest), University of California San Francisco Children’s Hospital, Schneider Children’s Hospital, Vanderbilt Children’s Hospital, and Wake Forest University Baptist Medical Center. The institutional review board at each center approved the protocol and written informed consent was obtained from parents before randomization. Study subjects were randomized to head cooling for 72 h starting within 6 h of birth, with rectal temperature maintained at 34.5 ± 0.5 °C (n = 116), followed by rewarming over 4 h, or standard care at 37.0 ± 0.5 °C (n = 118). The primary unfavorable study outcome was death or severe disability (Gross Motor Function Classification System level 3–5, Bayley II mental developmental index less than 70, or bilateral cortical visual impairment) at 18 mo. CoolCap Study subjects were eligible for the current analysis if they had moderate or severe HIE, with PCO2 levels recorded in the first 72 h of the study, and complete follow-up data (n = 196; Figure 1 ).
Temperature-corrected arterial blood gases, including PCO2, at prespecified time points (0, 4, 8, 12, 24, 48, and 72 h from study randomization) were analyzed to investigate the association between hypocapnia with the primary unfavorable outcome. Demographic and perinatal data collected included birth weight, gestational age, gender, race, mode of delivery, pregnancy complications, and Apgar scores at 1 and 5 min. Other clinical data were first pH, Sarnat stage at randomization (16), age at study randomization, treatment with hypothermia or standard therapy, presence of seizures, Bayley Scales of Infant Development II mental developmental index scores, and death at 18 mo. Infants were randomized at a mean 4.7 (range 2.1–6.1) hours after birth in the CoolCap Study (14). All times refer to time after randomization.
Statistical Analysis
Two sample t-tests for continuous variables, or Chi-square or Fisher’s exact test for categorical variables were used to compare demographic and neonatal characteristics of infants with unfavorable vs. favorable primary outcome. The relationship between hypocapnia and death/severe neurodevelopmental disability (primary outcome variable) was evaluated using a generalized additive model adjusting for the following variables: pH, neurological status at randomization (amplitude integrated electroencephalogram background and seizures: best prognosis group (normal or mildly abnormal with seizure); intermediate prognosis group (moderately abnormal with/without seizure AND severely abnormal without seizure); and worst prognosis group (severely abnormal with seizure)), birth weight quartile, Apgar score at 5 min less than 5, head cooled/standard care status, and Sarnat stage at randomization. This strategy allowed for incorporation of nonlinear forms of the predictors, namely pH and PCO2 in the generalized additive model, in addition to categorical explanatory variables and random effects for subjects. A thin plate regression spline technique was used as smoothing function. Model building was carried out including nonparametric smoothed functions of continuous variables as well as parametric forms. The minimal adequate model was selected by progressively deleting terms from a maximal model, minimizing residual deviance, using the chi-squared test as the deletion test. The minimal adequate model was used to predict probabilities of unfavorable outcome adjusting all remaining explanatory variables in the model other than the variable of interest. For all tests, P value < .05 was considered statistically significant. Statistical analyses were performed using R statistical and programming language (version 3.1.2); R package “mgcv” was used for the generalized additive model. For comparison of PCO2 at different time-points, ANOVA followed by Tukey’s test for post hoc comparisons was used.
The Coolcap Study Group
Executive Committee: P.D. Gluckman (chair, co-principal investigator), J.S. Wyatt (co-principal investigator), and A.J. Gunn (Scientific Officer). SAC: J.S. Wyatt (chair), R. Ballard, A.D. Edwards, D.M. Ferriero, P.D. Gluckman, A.J. Gunn, R. Polin, C. Robertson, and A. Whitelaw. Data Safety Committee: R. Soll (chair), M. Bracken, C. Palmer, M. Heymann, and A.Wilkinson. Hospital Investigators: J.R. Kaiser (Arkansas Children’s Hospital, 11 patients), M. Battin, D. Armstrong (University of Auckland-National Women’s Hospital, NZ, 11 patients), J. Khan (Children’s Memorial Hospital and Prentice Women’s Hospital of Northwestern Memorial Hospital, 3 patients), T. Raju (University of Illinois at Chicago, 1 patient), R. Polin, R. Sahni, U. Sanocka (Children’s Hospital of New York-Presbyterian, Columbia University, 18 patients), A. Rosenberg, J. Paisley (Children’s Hospital of Denver, 23 patients), R. Goldberg, M. Cotton (Duke University, 14 patients), A. Peliowski, E. Phillipos (Royal Alexandra Hospital/University of Alberta Hospital, 20 patients), D. Azzopardi, A.D. Edwards (Hammersmith Hospital, London, UK, 1 patient), F. Northington (Johns Hopkins University, 2 patients), J. Barks, S. Donn (University of Michigan-Mott Children’s Hospital, 12 patients), B. Couser (Children’s Hospital and Clinics of Minneapolis, 16 patients), D. Durand (Children’s Hospital and Research Center at Oakland, 8 patients), K. Sekar (Children’s Hospital of Oklahoma, 4 patients), D. Davis, M. Blayney (Children’s Hospital of Eastern Ontario/The Ottawa Hospital, 1 patient), S. Adeniyi-Jones (AI Dupont Children’s Hospital at Thomas Jefferson University, 6 patients), T. Yanowitz (Magee Women’s Hospital/ Children’s Hospital of Pittsburgh, 10 patients), R. Guillet, N. Laroia (Golisano Children’s Hospital at Strong, 10 patients), N. Finer, F. Mannino (University of California San Diego Medical Center (Hillcrest), 8 patients), J. Partridge (University of California San Francisco Children’s Hospital, 2 patients), D. Davidson (Schneider Children’s Hospital, 14 patients), A. Whitelaw (Southmead Hospital. Bristol, UK, 13 patients), M. Thoresen (St. Michael’s Hospital, Bristol, UK, 8 patients), J.S. Wyatt, F. O’Brien (University College Hospital, London, UK, 4 patients), B. Walsh (Vanderbilt Children’s Hospital, 13 patients), J. Perciaccante, and M. O’Shea (Wake Forest University Baptist Medical Center, 1 patient).
Manufacturer’s Representatives: J. Jones, T. Weiler, J. Mullane, D. Hammond, and J. Parnell (Olympic Medical, Seattle, WA).
Statement of Financial Support
No financial assistance was received to support this study
Disclosure
The authors have no real or perceived conflicts of interests to disclose or have any competing financial interests.
References
Kety SS, Schmidt CF. Effects of alterations in the arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. Fed Proc 1946;5(1 Pt 2):55.
Fritz KI, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of moderate hypocapnic ventilation on nuclear DNA fragmentation and energy metabolism in the cerebral cortex of newborn piglets. Pediatr Res 2001;50:586–9.
Giannakopoulou C, Korakaki E, Manoura A, et al. Significance of hypocarbia in the development of periventricular leukomalacia in preterm infants. Pediatr Int 2004;46:268–73.
Kubota M, Matsuda F, Hashizume M, Nakamura T, Nishida A. Periventricular leukomalacia associated with hypocarbia. Acta Paediatr Jpn 1996;38:57–60.
Liao SL, Lai SH, Chou YH, Kuo CY. Effect of hypocapnia in the first three days of life on the subsequent development of periventricular leukomalacia in premature infants. Acta Paediatr Taiwan 2001;42:90–3.
Murase M, Ishida A. Early hypocarbia of preterm infants: its relationship to periventricular leukomalacia and cerebral palsy, and its perinatal risk factors. Acta Paediatr 2005;94:85–91.
Okumura A, Toyota N, Hayakawa F, et al. Cerebral hemodynamics during early neonatal period in preterm infants with periventricular leukomalacia. Brain Dev 2002;24:693–7.
Okumura A, Hayakawa F, Kato T, et al. Hypocarbia in preterm infants with periventricular leukomalacia: the relation between hypocarbia and mechanical ventilation. Pediatrics 2001;107:469–75.
Shankaran S, Langer JC, Kazzi SN, Laptook AR, Walsh M ; National Institute of Child Health and Human Development Neonatal Research Network. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics 2006;118:1654–9.
Wiswell TE, Graziani LJ, Kornhauser MS, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996;98:918–24.
Fujimoto S, Togari H, Yamaguchi N, Mizutani F, Suzuki S, Sobajima H. Hypocarbia and cystic periventricular leukomalacia in premature infants. Arch Dis Child 1994;71:F107–10.
Klinger G, Beyene J, Shah P, Perlman M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch Dis Child Fetal Neonatal Ed 2005;90:F49–52.
Pappas A, Shankaran S, Laptook AR, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr 2011;158:752–758.e1.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Jensen EC, Bennet L, Hunter CJ, Power GC, Gunn AJ. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol 2006;572(Pt 1):131–9.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705.
Mirro R, Lowery-Smith L, Armstead WM, Shibata M, Zuckerman SL, Leffler CW. Cerebral vasoconstriction in response to hypocapnia is maintained after ischemia/reperfusion injury in newborn pigs. Stroke 1992;23:1613–6.
Bisschops LL, Hoedemaekers CW, Simons KS, van der Hoeven JG. Preserved metabolic coupling and cerebrovascular reactivity during mild hypothermia after cardiac arrest. Crit Care Med 2010;38:1542–7.
Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics 1995;95:868–74.
Vannucci RC, Brucklacher RM, Vannucci SJ. Effect of carbon dioxide on cerebral metabolism during hypoxia-ischemia in the immature rat. Pediatr Res 1997;42:24–9.
Zhou Q, Cao B, Niu L, et al. Effects of permissive hypercapnia on transient global cerebral ischemia-reperfusion injury in rats. Anesthesiology 2010;112:288–97.
Tao T, Zhao M, Yang W, Bo Y, Li W. Neuroprotective effects of therapeutic hypercapnia on spatial memory and sensorimotor impairment via anti-apoptotic mechanisms after focal cerebral ischemia/reperfusion. Neurosci Lett 2014;573:1–6.
Sandroni C, D’Arrigo S. Management of oxygen and carbon dioxide pressure after cardiac arrest. Minerva Anestesiol 2014;80:1105–14.
Kofstad J. Blood gases and hypothermia: some theoretical and practical considerations. Scand J Clin Lab Invest Suppl 1996;224:21–6.
Acknowledgements
We thank the many technicians, nurses, physicians, and scientists in the participant sites who contributed to the development and implementation of the CoolCap Study, and the parents who consented to enrollment of their infants in the trial who trusted in us under conditions of great stress and anxiety. We thank the many charities and research funding agencies who supported the preliminary research necessary for the study. The original study was designed by and was the responsibility of the Scientific Advisory Committee (SAC), who had full access to the trial data, and after reading and editing this manuscript, approved the final draft for submission.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Lingappan, K., Kaiser, J., Srinivasan, C. et al. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr Res 80, 204–208 (2016). https://doi.org/10.1038/pr.2016.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.62
This article is cited by
-
Neuroprotective therapies in the NICU in term infants: present and future
Pediatric Research (2023)
-
Transcutaneous carbon dioxide monitoring during therapeutic hypothermia for neonatal encephalopathy
Pediatric Research (2022)
-
Carbon dioxide levels in neonates: what are safe parameters?
Pediatric Research (2022)
-
Hypocapnia in early hours of life is associated with brain injury in moderate to severe neonatal encephalopathy
Journal of Perinatology (2022)
-
Non-invasive carbon dioxide monitoring in neonates: methods, benefits, and pitfalls
Journal of Perinatology (2021)