Abstract
Retinopathy of prematurity is a disease commonly affecting extremely premature babies. Indomethacin is widely used in the perinatal period. The goal of the present study was to test the hypothesis that indomethacin will improve retinopathy in a mouse model when administered during the period of injury (hyperoxia exposure) to the developing retinal vasculature. C57BL6 mice pups were exposed to 75% oxygen from postnatal d 7 through 12. Indomethacin was administered along with the oxygen exposure as a single subcutaneous dose of 0.5 mg/kg/d for 5 d. Animals were killed on postnatal d 17 through 20. The severity of retinopathy was assessed by a retinopathy scoring system of fluorescein-conjugated dextran-perfused retinal flat mounts and by quantitation of extraretinal nuclei by use of periodic acid-Schiff-stained retinal sections. Animals that received indomethacin during hyperoxia exposure had a significantly lower median (25th, 75th quartile) retinopathy score 5 (4.5, 6) compared with animals that received oxygen [8 (7.5, 10)]. Animals given indomethacin during hyperoxia exposure had a significantly lower extraretinal nuclei count per section (13.3 ± 4.6) (mean ± SD) compared with animals that were oxygen exposed (41.9 ± 14.7). Indomethacin did not affect the normal development of the retinal vasculature or the growth of the animals. The data show that indomethacin improves oxygen-induced retinopathy when administered concurrently with the injury phase without affecting the normal retinal development or growth of the animals.
Similar content being viewed by others
Main
ROP is a developmental vascular disease occurring in the incompletely vascularized retina of the premature infant(1). It can lead to a wide range of visual deficits including myopia, strabismus, amblyopia, severe visual impairment, and blindness. Many investigators have attempted to assess the effect of indomethacin on ROP. The results of these studies have been varied. In the National Collaborative Study to assess the efficacy of indomethacin in nonsurgical closure of PDA in a randomized trial, babies (n = 421) who had received indomethacin had a lower rate of ROP(2). Other smaller studies have found no effect (positive or negative) on the incidence or severity of ROP(3–6). Prociany et al.(3) found no difference in ROP in a retrospective study of 63 infants, 15 of whom were treated with indomethacin for PDA at a mean of 11 d of age. Yeh et al.(4) also found no difference in ROP in a randomized study of 47 infants. The authors acknowledge that this study was small. Bolling et al.(5) reported no effect of indomethacin on ROP in a study describing 31 infants. Bandstra et al.(6) reported no difference in ROP outcome in the use of a lower dose (0.1 mg/kg) of indomethacin in a prospective study to assess intraventricular hemorrhage outcome. Thus, clinical results regarding indomethacin and ROP are varied, but the National Collaborative Study(3), which is the largest, showed a beneficial effect.
Indomethacin is a nonsteroidal anti-inflammatory agent. It acts by inhibiting prostaglandin production. COX catalyzes the first committed step of arachidonic acid metabolism in the synthesis of prostaglandin. There are two isoforms of COX. COX-1 is expressed in most tissues and is responsible for physiologic production of prostaglandin. COX-2 is induced in the inflammatory cells and is responsible for production of prostaglandins during inflamation. Indomethacin inhibits both isoforms of COX(7). The prostaglandin cascade has been implicated in retinopathy and maintenance retinal blood vessel tone(8–14). In addition, indomethacin has been shown to inhibit subretinal neovascularization(13). Aspirin, a prostaglandin inhibitor, was found to increase the severity of retinopathy in a canine model(15).
Due to the varied effects of prostaglandin inhibitors on retinopathy and the importance of the prostaglandin cascade in retinopathy, the present study was performed to assess the effects of indomethacin on OIR, using a previously described mouse model(16).
METHODS
Animal model. The present study was approved by the Georgetown University Animal Care and Use Committee. C57BL6 mice were obtained from a commercial vendor (Taconic Laboratories, Germantown, NY). Newborn mice pups were maintained with their nursing mother. Pups along with their nursing mother were placed in 75 ± 2% oxygen for 5 d to produce an OIR as previously described and subsequently killed at P17 to 21, the time of greatest neovascular response(16). Exposure of the C57BL6 mice at P7 to 75% oxygen for 5 d produced a reproducible and quantifiable retinopathy, whereas earlier exposure produced dilated hyaloid vessels and a neovascular response that were not quantitatively reproduced(16).
Indomethacin experiments. The animals were divided into four different groups: room air, hyperoxia, room air with indomethacin, and hyperoxia with indomethacin. Indomethacin (Merck & Co., Inc., West Point, PA) was freshly prepared each day and used within 1 h of reconstitution. The drug was given in a single dose of 0.5 mg/kg/d administered subcutaneously in the nape of the neck. This dose of indomethacin was chosen to simulate total daily dose used clinically to treat a PDA of 0.4-0.5 mg/kg/d (i.e. 0.2 or 0.25 mg/kg every 12 h) as previously described in the National Collaborative Study(2). In addition, indomethacin has been used at concentration of 0.5 to 2 mg/kg in a rodent (rat) ulcer model in which prostaglandin effects were noted(17). Thus, 0.5 mg/kg/d was used in our experiments.
The drug therapy was started on P7 and given for 5 d to coincide with the period of oxygen exposure. The first dose of indomethacin was given just before placement in oxygen, and subsequent doses were administered while the animals remained in oxygen. The incubator was purged with oxygen while the animals received their daily injection to ensure the continuous high oxygen concentration. The animals were returned to room air on P12 and were killed with a lethal dose of pentobarbital from P17 through 20. The greatest neovascular response is maximal at P17-21 as assessed by neovascular tufts and extraretinal neovascular nuclei on retinal cross-sections(16). The retinal vasculature of these animals was studied by fluorescein-conjugated dextran angiography(18) by the retinopathy scoring system(19) or by quantification of extraretinal neovascularization by counting the extraretinal nuclei beyond the inner limiting membrane(16) on PAS-stained retinal sections(20).
Fluorescein dextran perfusion of the retinal blood vessels. To study the retinal vascular pattern, retinal flat mounts were performed using a perfusion of high molecular-weight dextran (2 000 000) conjugated with fluorescein (Sigma Chemical Co., St. Louis, MO) as previously described(18). Briefly, the animals were given sodium pentobarbital (120 mg/kg) and a median sternotomy was subsequently performed. The left ventricle was identified and 1 mL of a 50-mg/mL solution of high molecular-weight fluorescein-conjugated dextran with 4% paraformaldehyde was injected using a 27-gauge needle on a 1-mL tuberculin syringe. The eyes were then enucleated and placed in 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO) in PBS. The retinae were dissected using a dissecting microscope, placed on a glass slide with gelatin, and covered with a coverslip that was sealed with clear nail polish. Retinal scoring was then done in a masked fashion by at least two independent observers who used the retinopathy scoring system shown in Table 1(19).
Retinal sections. After the mice were killed with a lethal pentobarbital injection, the chest was opened and the left ventricle perfused with 4% paraformaldehyde in PBS. The eyes were then enucleated, placed in optimum cutting temperature-embedding compound (Sakura Fine Tek, Torrance, CA), and quickly frozen to -70°C. Serial sections 7-10 µm thick were then cut sagitally, parallel to the optic disc. The sections were stained with PAS stain(20). Extraretinal neovascularization was assessed by counting the number of nuclei from the blood vessels extending into the vitreous beyond the inner limiting membrane of the retina. Multiple sections from each eye were scored in a masked fashion by light microscopy (Nikon TMS). A minimum of six sections at least 50 µm apart were counted per animal, an average was obtained, and the average was used in the statistical analysis.
Animal growth. The weight of the mouse pups was checked with a laboratory balance. This was done on P7 and 12 and on the day the pups were killed, i.e. P17-20.
Statistical analysis. ANOVA by the Kruskal-Wallis test was performed to test for differences between the various treatment groups. Mann-Whitney tests by the Bonferroni method were used to compare the total retinopathy scores and retinopathy subscores. Total number of extraretinal nuclei and animal weight were compared using the t test. Statistical significance was defined as p < 0.05. Interobserver variability for retinal scoring-system data were determined using Kendall's τ-β correlation coefficient.
RESULTS
Retinopathy scoring system. Animals exposed to hyperoxia (n = 14) had a median total retinopathy score of 8 (7.5, 10) versus 1 (1, 1) (p < 0.001) in the control animals (n = 11). Thus, 5 d of 75% oxygen exposure caused a significant increase in the retinopathy scores. Indomethacin administered at a dose of 0.5 mg/kg/d (n = 15) concurrently with the 75% oxygen exposure improved the retinopathy score significantly (p < 0.001) when compared with oxygen-exposed animals [total retinopathy score, 5 (4.5, 6)]. There was no difference in the retinopathy scores of the animals given indomethacin while in room air [n = 9; score, 1 (1, 1)] compared with the control animals [n = 11; score, 1 (1, 1.5)], as shown in Figure 1. Figure 2 shows representative retinal whole mounts. Within each of the groups, the day the animals were killed, i.e. P17-20, did not affect the score. Nineteen animals were killed on P17 (six control, five control with indomethacin, five oxygen, and three oxygen with indomethacin), five on P18 (three oxygen and two oxygen with indomethacin), 21 on P19 (one control, four control with indomethacin, six oxygen, and 10 oxygen with indomethacin), and four on P20 (four control).
Total retinopathy scores. Animals exposed to hyperoxia had significantly higher total retinopathy scores (median ± 25th, 75th quartile) than animals administered indomethacin during hyperoxia (p < 0.001). There was no difference in the retinopathy score of animals in room air regardless of indomethacin use. ·Indicates control animals; ○indicates oxygen-treated animals.
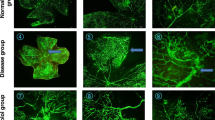
Retinal whole mounts showing the beneficial effect of indomethacin on OIR. A, Retina from a control animal (P17) shows normal vasculature characterized by fine vascular pattern throughout the retina. B, Retina from an animal exposed to hyperoxia (P18) shows an engorged tortuous appearance of the blood vessels, loss of central vasculature, and presence of blood vessel tufts. C, Retina from an animal given indomethacin concurrently with oxygen exposure (P17) shows central vasoconstriction. The vasculature looks less engorged with less tuft formation compared with the hyperoxia retina.
On comparison of the subscores in various categories, it was found that the animals that were given indomethacin concurrently with the oxygen administration had improvement in vascular tuft formation, extraretinal neovascularization, hemorrhage, and plus disease or tortuosity of the vessels compared with animals that did not receive indomethacin (Table 2). No differences were observed in the categories of central vasoconstriction or blood vessel growth.
Retinopathy scoring done by the investigators (B.N., T.R., K.Y., R.H.) had a significant interobserver correlation at the 0.01 level as determined by Kendall's τ-β test (Fig. 3).
Correlation of interobserver variability. Observer 1 scores are plotted on the x axis and observer 2 scores on the y axis. The points on the line show identical scores. Duplicated scores (n = 63) appear as a single point (i.e. observer 1 score of 0, observer 2 score of 2; or same scores, i.e. 1 and 1). Correlation was highly significant at the 0.01 level.
Quantification of extraretinal nuclei. When retinal sections stained with PAS to count the number of nuclei extending beyond the internal limiting membrane of the retina were examined, a significant difference (p < 0.05) was found in the number of extraretinal nuclei in animals that received indomethacin concurrently with hyperoxia exposure compared with those that did not (n = 6 for each group, 13.3 ± 4.6 versus 41.9 ± 14.7), as shown in Figure 4. This corroborated the findings of the scoring-system data, that there was significantly less extraretinal neovascularization in the oxygen-exposed animals receiving indomethacin. There was no significant difference in the nuclei count of the animals not exposed to hyperoxia regardless of the administration of indomethacin (4.7 ± 2.9 versus 3.4 ± 1.1, n = 5 for control and n = 7 for indomethacin).
Effect of indomethacin on extraretinal neovascularization. Animals that received indomethacin concurrently with the hyperoxia exposure had a significantly lower nuclei count (13.3 ± 4.6 vs 41.9 ± 14.7). Indomethacin did not affect the nuclei count of animals in room air (3.4 ± 1.1 vs 4.7 ± 2.9). · Indicates control animals; ○ indicates oxygen-treated animals.
Animal growth. The animals were weighed on P7 and 12 and at the time they were killed (P17-20). There was no significant difference in the weight-gain pattern between the control animals and the animals exposed to hyperoxia and indomethacin. In addition, because the day they were killed varied slightly (P17-20), weight gain per day from d 12 to that day was evaluated for each group and found not to differ (Table 3). Thus, neither indomethacin nor oxygen exposure affected the growth of the animals (Table 3).
DISCUSSION
Indomethacin improves OIR without adversely affecting the normal vascular development in the mouse. Indomethacin administration during hyperoxia exposure significantly lowered the total retinopathy scores. It improved the scores in the categories of blood vessel tufts, tortuosity, extraretinal neovascularization, and hemorrhage. There was no difference in the total retinopathy score or the subscores of animals that were in room air regardless of indomethacin administration. Animals given indomethacin during hyperoxia exposure had a significantly lower number of neovascular nuclei compared with animals that did not receive indomethacin concurrently with the hyperoxia. Indomethacin exposure did not affect the growth of the animals.
Various clinical studies have shown differing effects of indomethacin on ROP. Many studies show no effect of indomethacin on ROP(3–6); the National Collaborative Study, which was the largest prospective randomized published study, showed a lower rate of cicatricial ROP in indomethacin-treated infants(2). A role for the metabolites of arachidonic acid in the pathogenesis of OIR has been explored by many investigators(7–14). Because indomethacin is a nonspecific COX inhibitor, inhibiting both COX-1 and COX-2(7), it follows that it may prevent the neovascularization in retinopathy. Indomethacin can inhibit prostaglandin synthetase systems in the ocular tissues(8). The antiangiogenic properties of indomethacin in preventing subretinal neovascularization in the monkey model has been shown by Sakamoto et al.(13). In this monkey model, reduced inflammation as evidenced by less accumulation of macrophages in indomethacin-treated eyes was observed.
Aspirin has been shown to increase the severity of retinopathy in the beagle puppy model. In contrast, our results suggest a beneficial effect of indomethacin. The difference may be species related. The beagle was exposed to 100% oxygen for 72 h at d 2 of life, compared with the mouse that was exposed to 75% oxygen for 5 d at d 7 of life. In addition, the aspirin in the beagle model showed vasodilation. We observed no difference in indomethacin and oxygen-treated versus only oxygen-treated animals with respect to central vasoconstriction.
It is possible that a beneficial effect of indomethacin may also be time dependent, i.e. early administration may protect the vessels from oxidative stress damage. Early postnatal treatment with indomethacin reduced intraventricular hemorrhage in very low birth weight infants(21). No ROP outcome was reported in this study. It was postulated that indomethacin acts by scavenging prostaglandin-mediated free radicals(21). In addition, treatment of a beagle puppy model has shown that indomethacin accelerates germinal matrix maturation due to increased laminin deposition in basement membranes of matrix blood vessels(22). It is possible that indomethacin may have similar effects on retinal blood vessels and may stabilize fragile capillary beds.
In conclusion, the present study shows that indomethacin improves retinopathy when given concurrently with oxygen in a mouse model. We speculate that improvement of retinopathy was observed due to blockade of the COX pathway. This blockade results in the prevention of prostaglandin-mediated formation of free-radicals, thus preventing oxidative tissue injury. Indomethacin may act as a scavenger for free radicals and limit further tissue injury. Indomethacin may have a beneficial effect on OIR because of its effect on prostaglandin synthesis and, consequently, on retinal neovascularization.
Abbreviations
- P:
-
postnatal day
- PAS:
-
periodic acid-Schiff
- ROP:
-
retinopathy of prematurity
- OIR:
-
oxygen-induced retinopathy
- PDA:
-
patent ductus arteriosus
- COX:
-
cyclooxygenase
References
Phelps DL 1995 Retinopathy of prematurity. Pediatr Rev 16: 50–56
Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS 1983 Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr 102: 895–906
Procianoy RS, Garcia-Prats JA, Hittner HM, Adams JM, Rudolph AJ 1980 Use of indomethacin and its relationship to retinopathy of prematurity in very low birth weight infants. Arch Dis Child 55: 362–364
Yeh TF, Raval D, Pyati S, Pildes RS 1983 Retinopathy of prematurity (ROP) and indomethacin therapy in premature infants with patent ductus arteriosus (PDA). Prostaglandins 25: 385–391
Bolling J, Feman SS, Mellander M, Cotton R 1983 The influence of indomethacin on retinopathy of prematurity. Am J Ophthalmol 96: 254–255
Bandstra ES, Montalvo BM, Goldberg RN, Pacheo I, Ferrer PL, Flynn J, Gregorios JB, Bancalari E 1988 Prophylactic indomethacin for prevention of interventricular hemorrhage in premature infants. Pediatrics 82: 533–542
Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC 1996 Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 384: 644–648
Bhattacherjee P, Eakins KE 1974 Inhibition of the prostaglandin synthetase systems in ocular tissues by indomethacin. Br J Pharmacol 50: 227–230
Green K, Cheeks L, Luxenberg MN 1988 Topical indomethacin and prostaglandins in normal and aphakic rabbit eyes. Curr Eye Res 7: 1105–1111
Chemtob S, Roy MS, Abran D, Fernandez H, Varma DR 1993 Prevention of postasphyxial increase in lipid peroxides and retinal function deterioration in the newborn pig by inhibition of cyclooxygenase activity and free radical generation. Pediatr Res 33: 336–340
Hardy P, Abran D, Li DY, Fernandez H, Varma DR, Chemtob S. 1994 Free radicals in retinal and choroidal blood flow autoregulation in the piglet: interaction with prostaglandins. Invest Ophthalmol Vis Sci 35: 580–591
Chemtob S, Hardy P, Abran D, Li D, Peri K, Cuzzani O, Varma DR 1995 Peroxidase-cyclooxygenase interactions in postasphyxial changes in retinal and choroidal hemodynamics. J Appl Physiol 78: 2039–2046
Sakamoto T, Soriano D, Nassaralla J, Murphy TL, Oganesian A, Spee C, Hinton DR, Ryan SJ 1995 Effect of intravitreal administration of indomethacin on experimental subretinal neovascularization in the subhuman primate. Arch Ophthalmol 113: 222–226
Stuart MJ, Phelps DL, Setty BN 1988 Changes in oxygen tension and effects on cyclooxygenase metabolites: III. Pediatrics 82: 367–372
Flower RW, Blake D 1981 Retrolental fibroplasia: evidence for a role of the prostaglandin cascade in the pathogenesis of oxygen-induced retinopathy in the newborn beagle. Pediatr Res 15: 1293–1302
Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA 1994 Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35: 101–111
Shigeta J, Takahashi S, Okabe S 1998 Role of cyclo-oxygenase in the healing of gastric ulcers in rats. J Pharmacol Exp Ther 286: 1383–1390
D'Amato R, Weslowski E, Smith LE 1993 Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein-labeled dextrans in the mouse. Microvasc Res 46: 135–142
Higgins RD, Yu K, Sanders RJ, Nandgaonkar BN, Rotschild T, Rifkin DB 1999 Diltiazem reduces retinal neovascularization in a mouse model of oxygen-induced retinopathy. Curr Eye Res 18: 20–27
Bancroft JD, Cook HC, Sterling RW, Turner DP 1994 Manual of Histological Techniques and Their Diagnostic Application. Churchill Livingstone, Edinburgh, pp 134–136
Ment LR, Oh W, Ehrenkranz RA, Philip AGS, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJW, Katz KH, Schneider KC, Makuch RW 1994 Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 93: 543–550
Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA 1992 Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke 23: 1132–1137
Author information
Authors and Affiliations
Additional information
Supported in part by National Institutes of Health Grant EY00330.
Rights and permissions
About this article
Cite this article
Nandgaonkar, B., Rotschild, T., Yu, K. et al. Indomethacin Improves Oxygen-Induced Retinopathy in the Mouse. Pediatr Res 46, 184–188 (1999). https://doi.org/10.1203/00006450-199908000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199908000-00010
This article is cited by
-
Etanercept as a TNF-alpha inhibitor depresses experimental retinal neovascularization
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy
Pediatric Research (2016)
-
Risk Factors for Acute Retinopathy of Prematurity
Comprehensive Therapy (2007)
-
Risk Factors for Acute Retinopathy of Prematurity
Annals of Ophthalmology (2007)
-
Treatment of Retinopathy of Prematurity with topical ketorolac tromethamine: a preliminary study
BMC Pediatrics (2004)