Abstract
Amphiphilic triblock copolymers of poly(ethylene glycol) (PEG) and aliphatic polyesters such as poly(ɛ-caprolactone-co-glycolide) (PCGA) are typical examples of thermogelling polymers. In the gelation mechanism of these polymers, polymer chain transfer between the micelles, and subsequent aggregation of the micelles are important steps. We previously reported IP systems exhibiting temperature-responsive irreversible sol-gel transition that formed covalently cross-linked hydrogels. The IP formulation prepared by the ‘freeze–dry with PEG/dispersion’ method (D-sample) retained its sol state at r.t. longer than a formulation prepared by the usual dissolution method (S-sample). We hypothesized that polymer chain transfer between micelles was suppressed in the D-samples, because the micelle cores were in a solid-like state. In this study, we investigated polymer chain transfer by the fluorescence resonance energy transfer (FRET) method to reveal its role in the sol-to-gel transition. We synthesized a triblock copolymer of PCGA and PEG (tri-PCG) with attached naphthalene or dansyl groups at termini, tri-PCG-nap and tri-PCG-dan, as FRET donors and acceptors, respectively. The FRET behavior of the mixture of tri-PCG-nap/tri-PCG micelles and tri-PCG-dan/tri-PCG micelles was investigated. It was revealed that polymer chain transfer between micelles was strongly accelerated at the gelation temperature, and polymer chain transfer in D-samples was suppressed compared to S-samples.
Similar content being viewed by others
Introduction
Some types of polymer solutions exhibit sol-to-gel transitions in response to a temperature increase. Thermogelling polymer systems having sol-to-gel transition points between room temperature (r.t.) and body temperature have been extensively investigated as injectable polymer (IP) systems for biomedical applications. An aqueous solution of such a polymer is in the sol state at r.t. and can easily be injected into the body to form a hydrogel at the desired site. Biodegradable IP systems are expected to be applied clinically as implantable materials, because they can be excreted from the body after their expected roles are completed. Water-soluble bioactive reagents, such as proteins and peptides or living cells, can be mixed with the polymer solutions and injected at a desired site to form a hydrogel. Therefore, such biodegradable IP systems have been investigated for adapting to minimally invasive drug delivery systems (DDS) exhibiting sustained release of drugs,1, 2 biodegradable scaffolds in regenerative medicine,3, 4 materials for adhesion prevention after surgical operations,5, 6 embolization treatments of blood vessels7 and endoscopic submucosal dissection (ESD) materials.8 Amphiphilic block copolymers of poly(ethylene glycol) (PEG) as hydrophilic segments and biodegradable aliphatic polyesters as hydrophobic segments are known as typical examples of biodegradable IPs. For example, ABA triblock copolymers using poly(l-lactide) (PLLA),9 poly(d,l-lactide-co-glycolide) (PLGA),10, 11 poly(ɛ-caprolactone) (PCL),12, 13 and poly(ɛ-caprolactone-co-glycolide) (PCGA)14, 15, 16 as hydrophobic segments have been reported.
Such biodegradable thermogelling polymer systems have an advantage of rapid gelation in response to a temperature increase, however there have been some issues in previously reported biodegradable IP systems for clinical applications. The hydrogel once formed in the body is likely to revert to the sol state in a short period (typically <24 h) in the presence of a large amount of water or body fluid, such as in the intraperitoneal space or the interior of a blood vessel, because the gelation is caused by non-covalent (hydrophobic) interactions, and gel formation is an equilibrium process controlled by the surrounding conditions such as temperature and concentration. This may cause the disappearance of the hydrogel before it can perform its role or the rapid release of bioactive agents. Another problem is with the preparative processes for IP formulations. Most biodegradable IPs are sticky pastes in the dry state at r.t., and it takes a long time to dissolve them in aqueous solution. These characteristics are inconvenient in clinical settings and are obstacles for clinical applications of IP systems.
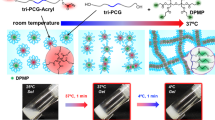
To solve these two problems, we recently reported biodegradable temperature-triggered covalent gelation systems exhibiting longer and controllable duration times of the gel state by a ‘mixing strategy’.17, 18 We synthesized a triblock copolymer of PCGA and PEG, PCGA-b-PEG-b-PCGA (tri-PCG), attaching acryloyl groups on both termini (tri-PCG-Acryl). A mixture of tri-PCG-Acryl micelle solution and tri-PCG micelle solution containing hydrophobic hexa-functional polythiols exhibited an irreversible sol-to-gel transition by covalent cross-linking in response to a temperature increase, and exhibited a longer and controllable duration time for the gel state.18 Just after mixing, the acryl groups of tri-PCG-Acryl in the micelle did not react with polythiol molecules because they existed in other micelle cores. Upon inter-micellar aggregation during the sol-to-gel transition, covalent cross-linking by the thiol-ene reaction of acryl groups of tri-PCG-Acryl with polythiol molecules occurred. Moreover, we applied the ‘freeze–dry with PEG/dispersion’ method19 as a quick extemporaneous preparation method developed in our previous studies on this system. We found that the IP formulations prepared by the ‘freeze–dry with PEG/dispersion’ method (D-samples) retained their sol state at r.t. after preparation longer (>24 h) than formulations prepared by the usual heating dissolution method (S-samples), which undergo gelation typically within 3 h after preparation.20 One of the differences between D-samples and S-samples is the state of the cores (solid-like or not). We confirmed that the micelle core of D-samples was in a solid-like semi-crystalline state. We hypothesized that gradual gelation in S-samples at r.t. is caused by transfer of tri-PCG-Acryl molecules from tri-PCG-Acryl micelles to tri-PCG micelles containing polythiols, and such polymer chain transfer is suppressed in D-samples. The polymer chain transfer rate for D-samples must be much slower than that for S-samples, because the micelle cores in D-samples were in a solid-like semi-crystalline state.
In this study, to confirm the hypothesis described above, we investigated the rate of polymer chain transfer (exchange) between micelles in the thermogelling polymer (tri-PCG) system. The previously proposed mechanism for sol-to-gel transition of biodegradable thermogelling polymer systems was a temperature-triggered micellar aggregation by dehydration of the PEG chain surrounding the micelles and subsequent growth of fibrous networks of the aggregation.17, 21, 22, 23, 24 However, the polymer transfer (exchange) behavior during the sol-to-gel transition has not been well investigated. Thus, we explore the effects of polymer transfer among micelles on the sol-to-gel transition, and reveal the primary factor for the stability of the D-sample.
To investigate polymer chain transfer in polymeric micelle systems, several methods have previously been reported, such as size-exclusion chromatography (SEC),25 fluorescence measurements,26, 27, 28 sedimentation29 and small-angle neutron scattering.30 In this study, we selected the fluorescence resonance energy transfer (FRET) method to investigate polymer chain exchange and transfer, because we can obtain real-time information using FRET for the polymer molecule transfer among the micelles in solution at various temperatures.
We employed naphthalene (nap) and dansyl (dan) groups as donors and acceptors, respectively, considering their overlapping fluorescence and absorption spectra31 and relatively small molecular sizes. These fluorescence groups were covalently attached to both termini of tri-PCG to give tri-PCG-nap and tri-PCG-dan. The obtained tri-PCG-nap and tri-PCG-dan were mixed separately with tri-PCG to prepare tri-PCG/tri-PCG-nap micelle solution and tri-PCG/tri-PCG-dan micelle solution. After mixing the two micelle solutions, the fluorescence spectra of the mixed solution under various conditions were measured to evaluate polymer chain transfer (Figure 1).
Materials and methods
Materials
PEG (molecular weight (MW)=1500 Da; PEG1500), ɛ-caprolactone (CL), tin 2-ethylhexanoate (Sn(Oct)2), N,N'-dicyclohexylcarbodiimide (DCC), and dimethylaminopyridine (DMAP) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). PEG (MW=2000 Da) (PEG2000) and glycolide (GL) were purchased from Sigma-Aldrich (St Louis, MO, USA). Dansyl glycine and 1-naphthoic acid were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Water was purified by a Milli-Q (Merck-Millipore, Darmstadt, Germany) system. All other reagents and organic solvents were commercial grade and used without further purification.
Measurements
1H nuclear magnetic resonance (1H-NMR) spectra were recorded on a nuclear magnetic resonance spectrometer (400 MHz, JNM-GSX-400, JEOL) using deuterated solvent (CDCl3 or D2O). The chemical shifts were calibrated against tetramethylsilane and/or solvent signal. The number-average molecular weights (Mn) were calculated from 1H-NMR spectra. The weight-average molecular weights (Mw) and the polydispersity indexes (Mw/Mn) of the polymers were determined by size exclusion chromatography (SEC) (column: TSKgel Multipore HXL-M × 2; detector: RI). The measurements were performed using dimethylformamide (DMF) as an eluent at a flow rate of 1.0 ml min−1 at 40 °C using a series of PEG as standards. The fluorescence spectra were recorded by a FP-8300 (JASCO, Tokyo, Japan) spectrofluorometer at 25 °C. The excitation wavelength was 290 nm with excitation and emission bandwidths of 5 nm. The spectra were recorded from 300 to 600 nm with a scan rate of 1000 nm min−1. Thermal analysis of the sample solutions was conducted using a differential scanning calorimeter (DSC-60, Shimadzu, Kyoto, Japan) with sealed aluminum pans.
Synthesis of tri-PCG-dan and tri-PCG-nap
PCGA-b-PEG-b-PCGA triblock copolymers (tri-PCG) were synthesized by ring-opening copolymerization of CL and GL in the presence of PEG1500 as a macroinitiator and Sn(Oct)2 as a catalyst according to the method reported previously19 (Supplementary Scheme S1). Briefly, PEG1500 (16.7 g, 11.16 mmol) in a 100- ml flask with a stopcock was dried under vacuum at 120 °C for 5 h. After cooling to r.t., CL (37.0 g, 324.5 mmol), GL (6.49 g, 55.9 mmol) (molar ratio of CL to GL (CL/GL) was 5.8) and Sn(Oct)2 (159.9 mg, 394.7 μmol) were added to the flask which was then dried under vacuum at r.t. for 12 h. Polymerization was carried out at 160 °C for 12 h by soaking the flask in an oil bath. The product was purified by reprecipitation using chloroform (100 ml) as a good solvent and diethyl ether (1000 ml) as a poor solvent to give a white solid of tri-PCG.
Tri-PCG-dan and tri-PCG-nap were synthesized through coupling reactions of terminal hydroxyl groups of tri-PCG with the carboxyl groups of dansyl glycine or 1-naphthoic acid (Scheme 1). Dansyl glycine (2.3 g, 7.47 mmol) was dissolved in DMF (7 ml). DCC (1.9 g, 8.98 mmol) was also dissolved in DMF (5 ml). These solutions were mixed and stirred at 4 °C for 1 h. The obtained solution was added to the solution of tri-PCG (5.0 g, 0.94 mmol) and DMAP (63.1 mg, 0.75 μmol) in DMF (15 ml). The mixture was then stirred for 48 h at r.t. Dicyclohexylurea produced as a byproduct was removed by filtration, and the filtrate was evaporated under reduced pressure to remove DMF. The obtained products were purified by reprecipitation using chloroform as a good solvent and a mixture of diethyl ether and methanol (4/1, v/v) as a poor solvent. The reprecipitation procedure was repeated several times until no low-molecular-weight compound was detected. The resulting precipitate was washed with a cooled mixture of diethyl ether and methanol (4/1, v/v), and dried under vacuum overnight to give tri-PCG-dan. Similar procedures were carried out using 1-naphthoic acid instead of dansyl glycine to give tri-PCG-nap.
Sol-to-gel transition of tri-PCG and mixture
The sol-to-gel transition behavior of tri-PCG and the mixtures was investigated by a test tube inverting method17 as follows. A vial containing a micelle solution was immersed in a water bath at the desired temperature for 15 min, removed from the water bath, then inverted repeatedly to determine sol state or gel state based on the criteria of ‘flow’ (=sol) and ‘no flow’ (=gel) in 30 s, with a temperature increment of 1 °C per step. Measurements were repeated three times at each temperature.
Preparation of samples for fluorescence measurements
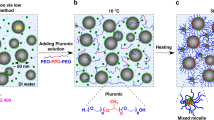
S-samples were prepared by the heating dissolution method as follows. Tri-PCG-dan (64.2 mg) and tri-PCG (1542 mg) were dissolved in acetone (64 ml; the weight ratio of tri-PCG-dan/tri-PCG=1/24). The obtained mixture (tri-PCG-dan/tri-PCG) solution was evaporated to remove acetone. Next, a predetermined amount of phosphate buffered saline (PBS) (pH=7.4, 10 mm) was added to the flask (total polymer concentration=0.025–15 wt%). After shaking with a vortex mixer for 1 min at r.t., the obtained suspension was heated to 65 °C and kept at 65 °C for 1 min, and further stirred by a vortex mixer for 1 min at r.t. The flask was immersed in ice-cold water for 2 min and further stirred by a vortex mixer for 1 min at r.t. These procedures were repeated until no insoluble particles were observed to give tri-PCG-dan/tri-PCG micelle solutions. Tri-PCG-nap/tri-PCG micelle solutions, where the weight ratio of tri-PCG-nap/tri-PCG was 0.7/24.3, were also prepared by the same procedure as for tri-PCG-dan/tri-PCG. Equal amounts of the obtained tri-PCG-dan/tri-PCG micelle solution and tri-PCG-nap/tri-PCG micelle solution were mixed together using a vortex mixer for 10 s. The resulting S-sample solution was transferred to a glass cuvette and incubated in a water bath at a designated temperature. After incubation for predetermined periods, fluorescence spectra were recorded at r.t. after 60 min incubation at r.t. unless otherwise noted.
D-samples were prepared by the freeze–dry with PEG/dispersion method as follows. Tri-PCG-dan (28.6 mg), tri-PCG (686.4 mg) and PEG2000 (71.5 mg) were mixed (weight ratio=1/24/2.5) and dissolved in acetone (7 ml). The solution was dissolved in pure water (70 ml) with stirring, then evaporated to remove acetone. The obtained aqueous solution was freeze-dried to give the mixture sample in powder form. A predetermined amount of PBS (pH=7.4, 10 mm) was added to the powder (total polymer concentration=15 wt%) in a test tube. The test tube was shaken by a vortex mixer for 1 min to give a macroscopically uniform milky dispersion. A dispersion of the mixture of tri-PCG-nap, tri-PCG and PEG2000 (weight ratio=0.7/24.3/2.5) was prepared by the same procedure. Equal amounts of the obtained tri-PCG-dan/tri-PCG/PEG2000 dispersion and tri-PCG-nap/tri-PCG/PEG2000 dispersion were mixed with stirring using a vortex mixer for 10 s to give D-samples. The resulting dispersion was transferred to a glass cuvette and incubated in a water bath at a designated temperature. After incubation for predetermined periods, fluorescence spectra were recorded at r.t. after 60 min incubation at r.t. unless otherwise noted.
TEM observation
The micelle morphology was observed by transmission electron microscopy (TEM) (JEOL JEM-1400) with acceleration voltage at 80 kV. The S-sample and D-sample for TEM were prepared by the same methods described above using pure water instead of PBS, and diluted with pure water to 2 wt%. An aliquot (4 μl) of the sample solution (2 wt%) was put on a STEM 150 Cu grid with hydrophilic polyvinylbutyral (PVB-C15) membrane (Okenshoji Co. Ltd., Tokyo, Japan). After 5 min incubation at r.t., the sample was dried by N2 gas, and negatively stained with 1 wt% hexaammonium heptamolybdate tetrahydrate aqueous solution followed by 1 min incubation and drying with N2 gas before observation.
Results and discussion
Synthesis of tri-PCG, tri-PCG-dan and tri-PCG-nap and sol-to-gel transition
The synthesis of tri-PCG-dan and tri-PCG-nap were successfully carried out from tri-PCG according to Scheme 1. The characterization results of tri-PCG, tri-PCG-dan and tri-PCG-nap are shown in Table 1. 1H-NMR spectra, SEC elution profiles and emission/excitation spectra of tri-PCG-dan and tri-PCG-nap are shown in the Supplementary Information (Supplementary Figures S1–S5). The purities of tri-PCG-dan and tri-PCG-nap were confirmed by SEC analysis (Supplementary Figure S4): no low-molecular-weight compounds were detected. The MW of tri-PCG-dan was higher than those of the other copolymers, because the low-molecular-weight fraction was removed during the reprecipitation process. The degree of substitution of dan and nap groups per terminal OH groups was calculated to be 71.6% and 97.0%, respectively, by H1-NMR integration. The introduction of the nap group was quantitative, but the dan group incorporation was not. The tri-PCG-dan sample contained about 50% of tri-PCG molecules having only one dan group, but this will not affect the investigation of polymer chain transfer by FRET. The mixing ratios of tri-PCG-dan and tri-PCG-nap to tri-PCG were 1/24 and 0.7/24.3 to adjust the molar ratio of dan/nap=1/1.
The tri-PCG solution (15 wt%) showed a sol-to-gel transition point at 35 °C (Supplementary Figure S6), and the mixtures of tri-PCG-dan/tri-PCG micelle solution and tri-PCG-nap/tri-PCG micelle solution (S-sample) showed the same (35 °C) sol-to-gel transition point as the tri-PCG solution. D-samples (mixture of tri-PCG-dan/tri-PCG micelles and tri-PCG-nap/tri-PCG micelles prepared by freeze–dry with PEG/dispersion method) showed sol-to-gel transition points at 36 °C.
Morphology of the micelles
To evaluate the morphology of the micelles in S-sample and D-sample, TEM observation of the sample solutions (or dispersions) was carried out. The results are shown in Figure 2. In Figure 2a, spherical particles with 40–60 nm diameters were observed for S-sample. The diameters were within reasonable level for flower-like polymeric micelles of tri-PCG. On the other hand, in Figures 2b and c, larger particles was observed for D-sample in addition to the similar size particles as seen in S-sample (Figure 2a). Some of the larger particles showed somewhat rugged shapes (Figure 2c). These results mean that some of D-sample micelles existed as aggregates and the other did not, showing good agreement with its turbid appearance.
FRET measurements on S-samples
The emission spectra of the S-samples (the mixture of tri-PCG-nap/tri-PCG micelle solution and tri-PCG-dan/tri-PCG micelle solution) after mixing at r.t. and further incubation at a predetermined temperature for 10 min were recorded. Figure 3a shows emission spectra of each sample before mixing, and Figure 3b shows typical examples of the emission spectra of diluted S-samples (copolymer concentration=0.025 wt%) with excitation wavelength at 290 nm after incubation at various temperatures. As the incubation temperature increased, the emission intensities at 370 nm assigned to the nap group gradually decreased, and the emission at 530 nm assigned to the dan group gradually increased. These results indicate the occurrence of FRET from the nap group to the dan group. FRET occurs when nap and dan groups exist in close proximity (Förster distance). These results suggest that the polymer chain (tri-PCG-dan or tri-PCG-nap) transfers between the micelles, and that the rate of polymer chain transfer is dependent on temperature. Therefore, the degree of polymer chain transfer in the mixed micelle solution can be evaluated by monitoring FRET.
(a) Emission spectra for each sample before mixing. Solid line: tri-PCG-nap/tri-PCG micelles (excitation at 290 nm); dotted line: tri-PCG-dan/tri-PCG micelles (excitation at 290 nm); dashed line: tri-PCG-dan/tri-PCG micelles (excitation at 370 nm). (b) Emission spectra for mixture of tri-PCG-nap/tri-PCG micelle solution and tri-PCG-dan/tri-PCG micelle solution (S-sample) after incubation at various temperatures for 10 min, and the additive spectrum of tri-PCG-nap/tri-PCG micelles (excitation at 290 nm) (solid line in a) and tri-PCG-dan/tri-PCG micelles (excitation at 290 nm) [dotted line in (a)]. Total copolymer concentration=0.025 wt%.
To evaluate the degree of polymer chain transfer (exchange) quantitatively, the emission intensity ratio for the peaks at 530 nm (dan) and 370 nm (nap) (I530/I370) was calculated as the polymer chain exchange index. Plots of I530/I370 as a function of incubation temperature are shown in Figure 4. The data for the homogeneous mixtures of tri-PCG-nap, tri-PCG-dan and tri-PCG-1 (weight ratio=0.7/1.0/48.3) are shown to estimate the FRET efficiency at the equilibrium state after complete polymer chain exchange in Figure 4. The emission intensity ratio I530/I370 for homogeneous samples was about 0.22 and remained constant with temperature variation, confirming that FRET efficiency was not dependent on temperature. We regarded I530/I370=0.217 (the average of observed values) as the theoretical maximum emission intensity ratio value where the degree of polymer chain exchange (Dex)=100%, and I530/I370=0.102 (theoretical value calculated from the additive spectra in Figure 3) as Dex=0%, as shown on the Y-axis on the right side in Figure 4. Therefore, Dex can be defined by the following equation:
Emission intensity ratio (I530/I370) and degree of polymer chain exchange for homogeneous mixture of tri-PCG-nap/tri-PCG-dan/tri-PCG micelles (○), and mixture of tri-PCG-nap/tri-PCG micelle solution and tri-PCG-dan/tri-PCG micelle solution (S-sample)  after incubation at various temperatures for 10 min. Total polymer concentration=0.025 wt%.
after incubation at various temperatures for 10 min. Total polymer concentration=0.025 wt%.

where Rt is the observed I530/I370 value, Rmax is the I530/I370 value for a homogeneous mixture (=0.217), and Rmin is the theoretical value of I530/I370 with no polymer exchange (=0.102).
I530/I370 (and Dex) for the mixture of tri-PCG-nap/tri-PCG micelle solution and tri-PCG-dan/tri-PCG micelle solution (S-sample) increased with increasing temperature. This means that the rate of polymer chain transfer was greater at higher temperature. Over 48 °C, the Dex value reached a plateau at about 87%. Furthermore, the graph was a sigmoidal curve with a threshold at 40–45 °C. In a diluted condition (0.025 wt%, sufficiently below gelation concentration), the tri-PCG solution showed a higher transition point over 40 °C, which was detected by a diameter change in dynamic light scattering (DLS; data not shown). These results suggest that the increase in Dex along with temperature was not only due to kinetic acceleration by the temperature increase, and the polymer transfer rate was significantly increased around the sol-to-gel transition temperature.
We also investigated the effects of polymer concentration on polymer chain transfer. Figure 5 shows the time course of the degree of polymer chain exchange (Dex) for various polymer concentrations after incubation at 35 °C. The emission spectra of the samples were recorded after dilution to 0.025 wt%, because the fluorescence intensity was too large when the concentration was high. Upon increasing polymer concentration, the Dex value reached a plateau in a shorter period of time. It is quite reasonable that the polymer exchange rate is higher at a higher polymer concentration. Mattice et al. reported that the polymer chain exchange rate was very slow at low polymer concentration27 and the mechanism of polymer chain exchange was suggested to be micellar merger/splitting at higher polymer concentration by dynamic Monte Carlo (MC) simulations.32 Our results showed good agreement with their hypothesis and provided evidence from an actual system that polymer chain exchange was promoted by merger and splitting between micelles at high polymer concentration. Therefore, we confirmed that polymer chain transfer behavior can be examined under higher (15 wt%) polymer concentration.
Figure 6 shows the time course of Dex for the mixture of tri-PCG-nap/tri-PCG micelle solution and tri-PCG-dan/tri-PCG micelle solution (S-sample) with 15 wt% polymer concentration at various incubation temperatures. The increase in the Dex value was much faster at higher incubation temperature. The rate of polymer chain transfer between the micelles strongly depended on temperature. Polymer chain transfer was very slow at 25 °C. On the basis of the results of DLS measurements, the diameter of the tri-PCG micelle was constant at 25 °C (Supplementary Figure S7). These results suggest that the micelles neither merged nor aggregated at 25 °C. At a temperature sufficiently lower than the transition point, PEG chains existing at the shell layer of the micelles were adequately hydrated and the hydrophobic cores of the micelles were not exposed to the aqueous phase by exclusion volume effects of the PEG chains. This is the reason why merger/aggregation of micelles hardly occurred at low temperature even at high polymer concentration. After 1 min incubation at 35 °C, the micelle solution underwent gelation, but the Dex value still increased for an additional 9 min after gelation. These results indicate that polymer chain transfer occurred even after gelation, and the polymer chains could move in the hydrogel. On the other hand, at 30 °C, below the sol-to-gel transition temperature, the Dex value also increased gradually. These results suggest that some polymer chain transfer occurred below the gelation temperature.
We found that the rate of polymer chain transfer was significantly accelerated around the gelation temperature. In previous reports, the sol-to-gel transition occurred by association of micelles,21, 22, 23, 24 and polymer chain transfer (exchange) occurred by merger between micelles.32 However, it was suggested that polymer chain transfer began before the gelation temperature was reached and continued after gelation.
Comparison of D-samples and S-samples
As described in the introduction, we previously discovered that the temperature-responsive covalent cross-linking IP formulations prepared by the ‘freeze–dry with PEG/dispersion’ method (D-samples) retained the sol state longer (more than 24 h) than the formulations prepared by the usual heating dissolution method (S-samples), which undergo gelation typically within 3 h after preparation (Supplementary Figure S8).20 We hypothesized that differences in stability (no gelation during incubation at r.t.) between D-samples and S-samples are due to differences in the rate of polymer chain transfer at r.t. To confirm this hypothesis, we investigated polymer chain transfer for both D-samples (15 wt%) and S-samples for an extended period of time at 25 °C. In this experiment, the emission intensity for the samples was measured immediately after dilution to 0.025 wt%. The results are shown in Figure 7. Clearly, the increase in the Dex value for the S-sample at the initial stage was significantly faster than that of the D-sample. After the initial (ca. 10 min) fast increase, the Dex value for the S-sample increased more gradually. For the D-sample, no rapid increase at the initial stage was observed and the Dex value increased gradually. After 180 min, the Dex values were about 45 and 20% for the S-sample and D-sample, respectively. Considering our previous results showing that spontaneous covalent gelation occurred in 3 h for S-samples,20 the critical degree of polymer exchange for gelation should exist between 20 and 45%. It was confirmed that the rate of polymer chain transfer in the S-sample was much faster than that in the D-sample at r.t especially at the initial stage. We confirmed that the D-sample showed an endothermic peak at 30 °C and the micelle core was in a solid-like semi-crystalline state by DSC measurements (Figure 8). Therefore, polymer chain transfer was suppressed in the D-sample because it was not in the usual equilibrium state between unimers and micelles. The initial stage polymer chain transfer was quite low for the D-sample because the amount of unimers in the D-sample micelle solution was extremely low. In addition, the unimers, if they existed, were difficult to incorporate into other micelles because the micelle cores were in the solid-like semi-crystalline state.
The main reason of the difference between S-samples and D-samples is the difference of the core states, which must be derived from the preparation process (freeze–dry or heating dissolution) as follows. D-sample was prepared by the freeze–dry with PEG/dispersion method. After freeze–dry, the core of the sample was partially crystalized. Although certain part of micelle was aggregated, the PEG molecules, as additives, existed between the micelles in the aggregates, which helped rapid dispersion of the D-samples. During this dispersion process, the temperature was kept below the melting temperature. So, the crystal formed in the core was not melted, and no water molecule could penetrate into the micelle core. As results, the cores of D-sample micelles kept their semi-crystalline state. On the other hand, during the process for S-sample preparation (heating dissolution), temperature was raised above the melting temperature, and the crystal in the core was melted. But, during the cooling process, recrystallization in the micelle core was not proceeded in the presence of solvent (water). So, the core of the S-sample micelle became amorphous state.
To estimate the degree of polymer chain exchange in S-samples and D-samples during the sol-to-gel transition process, the Dex value under continuous temperature increase (from 25 °C to 42 °C, 1 °C min−1) was monitored (Figure 9). The S-sample showed gelation at 35 °C, and its Dex value increased rapidly in the range of 30–37 °C. Thus, polymer chain transfer occurred in advance of gelation, and this result shows good agreement with the results shown in Figure 6. The percolation transition of associated micelles is considered to be the prevailing mechanism for the sol-to-gel transition. However, the association of micelles and polymer chain transfer began before reaching the transition temperature of percolation. In contrast, the Dex value of the D-sample gradually increased from 25 to 42 °C: no rapid increase at a certain temperature was observed. The D-sample showed gelation at 36 °C, where the Dex value was about 50%, which was significantly lower than that of the S-sample at the gelation temperature (78% at 35 °C). This result indicated that exchange of polymer chains between micelles is suppressed in D-samples compared to S-samples.
Conclusion
We successfully synthesized PCGA-b-PEG-b-PCGA with naphthalene or dansyl groups covalently attached at both termini, tri-PCG-nap and tri-PCG-dan, as FRET donors and acceptors, respectively. The FRET observations provided an estimation of polymer chain transfer between the micelles in the mixture of tri-PCG-nap/tri-PCG micelles and tri-PCG-dan/tri-PCG micelles (S-sample or D-sample). Polymer chain transfer between micelles for the S-sample was strongly dependent on the polymer concentration and temperature, and was accelerated at the gelation temperature. It was revealed that polymer chain transfer began in advance of gelation, and continued even after gelation. We previously reported that covalent cross-linking IP formulations prepared by the ‘freeze–dry with PEG/dispersion’ method (D-samples) retained the sol state at r.t. longer compared to S-samples prepared by the heating dissolution method, and hypothesized that polymer chain transfer between micelles was suppressed in D-samples, because the micelle cores were in a solid-like semi-crystalline state. In this study, we confirmed that polymer chain transfer in D-samples was in fact suppressed compared with S-samples. Therefore, the results obtained in this study strongly support our hypothesis.

Synthesis of tri-PCG-dan and tri-PCG-nap.
References
Choi, S., Baudys, M. & Kim, S. W. Control of blood glucose by novel GLP-1 delivery using biodegradable triblock copolymer of PLGA-PEG-PLGA in type 2 diabetic rats. Pharm. Res. 21, 827–831 (2004).
Li, K., Yu, L., Liu, X., Chen, C., Chen, Q. & Ding, J. A long-acting formulation of a polypeptide drug exenatide in treatment of diabetes using an injectable block copolymer hydrogel. Biomaterials 13, 1195–1203 (2013).
Yeon, B., Park, M. H., Moon, H. J., Kim, S., Cheon, Y. W. & Jeong, B. 3D culture of adipose-tissue-derived stem cells mainly leads to chondrogenesis in poly(ethylene glycol)-poly(L-alanine) diblock copolymer thermogel. Biomacromolecules 14, 3256–3266 (2013).
Nagahama, K., Ouchi, T. & Ohya, Y. Temperature-induced hydrogels through self-assembly of cholesterol-substituted star PEG-b-PLLA copolymers: an injectable scaffold for tissue engineering. Adv. Funct. Mater. 18, 1220–1231 (2008).
Zhang, Z., Ni, J., Chen, L., Yu, L., Xu, J. & Ding, J. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 32, 4725–4736 (2011).
Hong, J. H., Choe, J. W., Kwon, G. Y., Cho, D. Y., Sohn, D. S., Kim, S. W., Woo, Y. C., Lee, C. J. & Kang, H. The effects of barrier materials on reduction of pericardial adhesion formation in Rabbits: a temperature sensitive poloxamer solution/gel material. J. Surg. Res. 166, 206–213 (2011).
Weng, L., Rostambeigi, N., Zantek, N. D., Rostamzadeh, P., Bravo, M., Carey, J. & Golzarian, J. An in situ forming biodegradable hydrogel-based embolic agent for interventional therapies. Acta Biomater. 9, 8182–8192 (2013).
Yu, L., Xu, W. ei., Shen, W., Cao, L., Liu, Y., Li, Z. & Ding, J. Poly(lactic acid-co-glycolic acid)-poly(ethylene glycol)-poly(lactic acid-co-glycolic acid) thermogel as a novel submucosal cushion for endoscopic submucosal dissection. Acta Biomater. 10, 1251–1258 (2014).
Fujiwara, T., Mukose, T., Yamaoka, T., Yamane, H., Sakurai, S. & Kimura, Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA-PEG-PLLA and PDLA-PEG-PDLA. Macromol. Biosci. 1, 204–208 (2001).
Jeong, B., Bae, Y. H. & Kim, S. W. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules 32, 7064–7069 (1999).
Chen, C., Chen, L., Cao, L., Shen, W., Yu, L. & Ding, J. Effects of L-lactide and D,L-lactide in poly(lactide-co-glycolide)-poly(ethylene glycol)-poly(lactide-co-glycolide) on the bulk states of triblock copolymers, and their thermogellation and biodegradation in water. RSC Adv 4, 8789–8798 (2014).
Bae, S. J., Suh, J. M., Sohn, Y. S., Bae, Y. H., Kim, S. W. & Jeong, B. Thermogelling poly(caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules 38, 5260–5265 (2005).
Liu, C. B., Gong, C. Y., Huang, M. J., Wang, J. W., Pan, Y. F., Zhang, Y. D., Li, G. Z., Gou, M. L., Wang, K., Tu, M. J., Wei, Y. Q. & Qian, Z. Y. Thermoreversible gel-sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J. Biomed. Mater. Res. Part B 84, 165–175 (2008).
Jiang, Z., You, Y., Deng, X. & Hao, J. Injectable hydrogels of poly(ɛ-caprolactone-co-glycolide)-poly(ethylene glycol)-poly(ɛ-caprolactone-co-glycolide) triblock copolymer aqueous solutions. Polymer 48, 4786–4792 (2007).
Jiang, Z., You, Y., Gu, Q., Hao, J. & Deng, X. Effect of microstructures on the phase transition behavior of P(CL-GL)-PEG-P(CL-GL) triblock copolymer aqueous solutions. Macromol. Rapid Commun. 29, 1264–1268 (2008)
Yu, L., Sheng, W., Yang, D. & Ding, J. Design of molecular parameters to achieve block copolymers with a powder form at dry state and a temperature-induced sol-gel transition in water without unexpected gelling prior to heating. Macromol. Res. 21, 207–215 (2013).
Yoshida, Y., Kawahara, K., Inamoto, K., Mitsumune, S., Ichikawa, S., Kuzuya, A. & Ohya, Y. Biodegradable injectable polymer systems exhibiting temperature-responsive irreversible sol-to-gel transition by covalent bond formation. ACS Biomater. Sci. Eng 3, 56–67 (2017).
Yoshida, Y., Takai, H., Kawahara, K., Mitsumune, S., Takata, K., Kuzuya, A. & Ohya, Y. Biodegradable injectable polymer systems exhibiting longer and controllable duration time of the gel state. Biomater Sci. 5, 1304–1314 (2017).
Yoshida, Y., Takahashi, A., Kuzuya, A. & Ohya, Y. Instant preparation of a biodegradable injectable polymer formulation exhibiting a temperature-responsive sol-gel transition. Polym. J. 46, 632–635 (2014).
Takata, K., Yoshida, Y., Takai, H., Kawahara, K., Mitsumune, S., Kuzuya, A. & Ohya, Y. Extemporaneously preparative biodegradable injectable polymer systems exhibiting temperature-responsive irreversible gelation. J. Biomater. Sci. Polym. Ed. (e-pub ahead of print 23 May 2017; doi:10.1080/09205063.2017.1330114
Yu, L., Chang, G., Zhang, H. & Ding, J. Temperature-induced spontaneous sol-gel transitions of poly(D,L-lactic acid-co-glycolic acid)-b-Poly(ethylene glycol)-b-poly(D,L-lactic acid-co-glycolic acid) triblock copolymers and their end-capped derivatives in water. J. Polym. Sci. Polym. Chem. 45, 1122–1133 (2007).
Zhang, H., Yu, L. & Ding, J. Roles of hydrophilic homopolymers on the hydrophobic-association-induced physical gelling of amphiphilic block copolymers in water. Macromolecules 41, 6493–6499 (2008).
Yang, Z. & Ding, J. A thermosensitive and biodegradable physical gel with chemically crosslinked nanogels as the building block. Macromol. Rapid Commun. 29, 751–756 (2008).
Yu, L., Zhang, Z., Zhang, H. & Ding, J. Mixing a sol and a precipitate of block copolymers with different block ratios leads to an injectable hydrogel. Biomacromolecules 10, 1547–1553 (2009).
Yamamoto, Y., Yasugi, K., Harada, A., Nagasaki, Y. & Kataoka, K. Temperature-related change in the properties relevant to drug delivery of poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles in aqueous milieu. J. Controlled Release 82, 359–371 (2002).
Jule, E., Yamamoto, Y., Thouvenin, M., Nagasaki, Y. & Kataoka, K. Thermal characterization of poly(ethylene glycol)-poly(D,L-lactide) block copolymer micells based on pyrene excimer formation. J. Control. Release 97, 407–419 (2004).
Wang, Y., Balaji, R., Quirk, R. P. & Mattice, W. L. Detection of the rate of exchange of chains between micelles formed by diblock copolymers in aqueous solution. Polym. Bull. 28, 333–338 (1992).
Wang, Y., Kausch, C. M., Chun, M., Quirk, R. P. & Mattice, W. L. Exchange of chains between micelles of labeled polystyrene-block-poly(oxyethylene) as monitored by nonradiative singlet energy transfer. Macromolecules 28, 904–911 (1995).
Tian, M., Qin, A., Ramireddy, C., Webber, S. E. & Munk, P. Hybridization of block copolymer micelles. Langmuir 9, 1741–1748 (1993).
Lu, J., Bates, F. S. & Lodge, T. P. Remarkable effect of molecular architecture on chain exchange in triblock copolymer micelles. Macromolecules 48, 2667–2676 (2015).
Wu, P. & Brand, L. Resonance energy transfer: methods and applications. Anal. Biochem. 218, 1–13 (1994).
Haliloglu, T., Bahar, I., Erman, B. & Mattice, W. L. Mechanisms of the exchange of diblock copolymers between micelles at dynamic equilibrium. Macromolecules 29, 4764–4771 (1996).
Acknowledgements
This work was financially supported in part by Private University Research Branding Project: Matching Fund Subsidy from Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan (2016–2020), a Grant-in-Aid for Scientific Research (16H01854) from the Japan Society for the Promotion of Science (JSPS), and by the Kansai University Outlay Support for Establishing Research Centers, 2016.The author thank SC Organic Chemical Co. Ltd. for providing DPMP. We thank Dr Yuta Yoshizaki, Mr Hiroki Takai and Mr Takuya Nagata for their helping in additional experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Takata, K., Kawahara, K., Yoshida, Y. et al. Analysis of the sol-to-gel transition behavior of temperature-responsive injectable polymer systems by fluorescence resonance energy transfer. Polym J 49, 677–684 (2017). https://doi.org/10.1038/pj.2017.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.33
This article is cited by
-
Cluster growth from a dilute system in a percolation process
Polymer Journal (2020)
-
Temperature-responsive biodegradable injectable polymer systems with conveniently controllable properties
Polymer Journal (2019)
-
PJ ZEON Award for outstanding papers in Polymer Journal 2017
Polymer Journal (2018)








 , or 25 °C
, or 25 °C  . Total polymer concentration=15 wt%.
. Total polymer concentration=15 wt%.


 and D-sample (□) under continuous temperature increase (1 °C min−1). The polymer concentration is 15 wt%.
and D-sample (□) under continuous temperature increase (1 °C min−1). The polymer concentration is 15 wt%.