Abstract
To obtain a temperature-responsive CO2 absorbent with a high amine content, temperature-responsive gel particles (GPs) consisting of poly(N-isopropylacrylamide-co-polyvinylamine) (poly(NIPAm-co-VAm)) were designed and prepared. Stable poly(N-isopropylacrylamide) GPs with a high N-vinylformamide (NVF) content were prepared in aqueous media by optimization of the concentrations of the surfactant and monomers used in the polymerization step. GPs with a high polyvinylamine (pVAm) content up to 3.2 mmol amine per g GPs (~30 mol%) were prepared as a stable aqueous solution via the complete and selective hydrolysis of pNVF in the poly(NIPAm-co-NVF) GPs in a methanol solution, then dialyzed against water. The GPs with 3.2 mmol amine per g GPs pVAm showed a volume phase transition at a temperature of ~65 °C. Conductivity experiments established that the aqueous solution of the poly(NIPAm-co-VAm) GPs reversibly absorbed CO2 in response to a small thermal stimulus (30–75 °C). In addition, the foaming of amine-functionalized GP solutions during CO2 bubbling was prevented by increasing the amount of crosslinking in the GPs.
Similar content being viewed by others
Introduction
It is of great importance to decrease the CO2 emissions in the atmosphere, because CO2 is a major contributor to global climate change.1 Post-combustion CO2 separation from main emission sources, such as coal-fired power plants, is one of the most important techniques for CO2 capture and storage.2 Chemical absorption by an aqueous solution of low-molecular-weight amines, such as ethanol amines, is the most widely used method to selectively absorb CO2 from post-combustion gases through acid–base reactions.2, 3, 4, 5 However, the disadvantages of chemical absorption, such as volatilization of the low-molecular-weight amines, corrosion of the absorption tower and, most of all, the high energy consumption due to the high regeneration temperature of above 140 °C,2 have limited its further application.
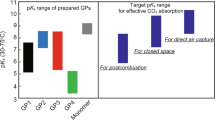
As an alternative to absorption by an aqueous solution of low-molecular-weight amines, adsorption by porous materials, such as zeolites, activated carbons, metal–organic frameworks and polymer amine-supported silica, has received much interest. CO2 can be selectively adsorbed onto the surface of solid sorbents via a physical or chemical interaction. Porous sorbents have shown a large CO2 capture capacity per unit mass, owing to their high specific surface area.6, 7, 8, 9, 10, 11 However, in the presence of water vapor, the CO2 adsorption capacity is occasionally decreased because liquefied water vapor blocks the capillaries and pores and/or competes with CO2 molecules for adsorption on the pore surface of adsorbents.12 Thus, porous sorbents are not always optimal for CO2 recovery from post-combustion exhaust gas, which contains 5–7% water vapor.13 Therefore, the development of a CO2 sorbent that is capable of working in an environment that contains water vapor and that can be regenerated at low temperatures (<100 °C) is of interest.
Aqueous solutions14 and films15, 16 of tertiary amine N-[3-(dimethylamino)propyl]methacrylamide (DMAPM)14, 15, 16- or secondary amine N-(2,2,6,6-tetramethylpiperidin-4-yl)methacrylamide (TMPMA)17-functionalized poly(N-isopropylacrylamide) (pNIPAm) micro-gel particles (GPs) can reversibly capture CO2 at approximately room temperature (30 °C) and efficiently release CO2 at elevated temperatures (75 °C). pNIPAm GPs swell and shrink reversibly at temperatures below and above the volume phase transition temperature (VPTT) as a result of the phase transition between the hydrophilic state and the hydrophobic state.18, 19 Consequently, the pKa value of amines within the GPs is high in the swollen state, owing to the low steric hindrance and high dielectric constant, whereas in the collapsed state, the pKa value decreases.14, 16 As a result, CO2 can be captured and released efficiently over a small temperature range.14, 16 However, the CO2 capture capacity per unit mass of polymer is not sufficiently high, because of the low amine content of pDMAPM or pTMPMA (5.9 and 4.5 mmol g−1, respectively).
Polyvinylamine (pVAm) is the polymer with the highest amine content per unit mass (23.8 mmol g−1). Pelton and Akashi et al.20, 21, 22, 23, 24 have reported that temperature-responsive pVAm can be obtained from a copolymer of N-vinylformamide (NVF) and N-isopropylacrylamide (NIPAm), p(NVF-co-NIPAm), which can be readily prepared via free radical copolymerization. The poly-N-vinylformamide (pNVF) units in the copolymer can be partially hydrolyzed under acidic or basic conditions to produce pVAm.20, 21, 22, 23, 24 However, owing to the difference in the reaction ratio between NIPAm (~1) and NVF (~0.09),25, 26 the reported incorporation percentage of NVF into the pNIPAm-based GPs is only 10%.24 Berkland et al.27 have reported a method to prepare GPs that consist of crosslinked pVAm, and although the pVAm ratio is quite high, the GPs are not capable of responding to temperature changes in the environment.
However, our previous study has shown that the foaming of amine-functionalized GP solutions during CO2 bubbling is a severe problem that inhibits their application. Increasing the degree of crosslinking in GPs is an effective method to abate foam formation and viscosity increase of polymer solutions.28 Therefore, GPs with not only a high incorporation rate of NVF but also a high degree of crosslinking were favored in this study.
In this paper, we report a procedure to synthesize temperature-responsive GPs that contain large amounts of the smallest amine unit, vinylamine (VAm). To maximize the VAm content and avoid the foaming of the solution, the polymerization conditions of GPs, such as the monomer feed ratio, total monomer concentration, surfactant concentration, crosslinker concentration and hydrolysis conditions, were optimized. The CO2 absorption behavior of the optimized GPs was also studied.
Experimental Procedures
Materials
NVF was obtained from Tokyo Chemical Industries and used after distillation. NIPAm (Wako Pure Chemical) was purified via recrystallization (benzene/hexane). N,N′-methylenebisacrylamide (BIS) (Tokyo Chemical Industries), the surfactant cetyltrimethylammonium bromide (CTAB, MP Biomedical, LLC, Tokyo, Japan) and the initiator 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPD, Wako Pure Chemical Industries, Osaka, Japan) were used as received. NaOH, KOH and methanol were obtained from Kanto Chemical (Tokyo, Japan). NaOCH3, HCl, H2SO4 and CF3COOH were obtained from Wako Pure Chemical. A dialysis membrane with a molecular weight cutoff of 12 000–14 000 (Thermo Fisher Scientific) was used to purify the GPs.
Synthesis of the NVF-containing GPs
According to the GP polymerization conditions described in Table 1, the monomer NVF, NIPAm and the crosslinker BIS were dissolved in water, thus resulting in a total monomer concentration of 312, 156 or 62.4 mM. The surfactant CTAB was added to the monomer solution, which was then degassed with nitrogen by stirring at 70 °C for 30 min. Then, an aqueous solution of the initiator AAPD (70 mg ml−1) was added. The polymerization was performed at 70 °C for 3 h under a nitrogen atmosphere to obtain NVF-containing GPs (NVF-GPs). The resulting mixture was purified via dialysis against an excess volume of water for 4 days (the water was changed more than three times a day) to remove unreacted monomers, oligomers and surfactants (Scheme 1).
Ion exchange
Anion exchange of the GPs was conducted to remove the trace amounts of counter anions and CO2 absorbed by the amines during dialysis. Briefly, 1 M HCl was slowly added to the dialyzed GP solution until the pH was lowered below 4 to release CO2. Then, an excess amount of activated anion-exchange resin was added to the solution during N2 purging. The resin was removed via filtration after the exchange process was conducted for longer than 1 h.
Proton nuclear magnetic resonance measurement
The compositions of the NVF-GPs and VAm-GPs were characterized by using (1H NMR) proton nuclear magnetic resonance spectroscopy. The lyophilized GPs were dissolved in methanol-d4 at a concentration of 10 mg ml−1.
Hydrodynamic diameter measurement
The hydrodynamic diameter of the synthesized GPs was measured by using dynamic light-scattering spectrophotometry (Zetasizer Nano ZS, Malvern, Malvern Instruments Ltd, Worcestershire, UK). A sample with a concentration of 0.1 mg ml−1 was set at each temperature for 2 min, and the measurements were performed three times. The diameter of the GPs after hydrolysis was measured at basic pH after anion exchange. The diameter of the CO2-absorbed GPs was also measured similarly after bubbling CO2 (10% CO2 and 90% N2) into an aqueous solution of VAm-GPs for 12 h at a flow rate of 10 ml min−1.
CO2 absorption–desorption measurement
The absorption of CO2 in solution can be monitored by measuring the increase in conductivity of the solution because HCO3−1 works as a charge carrier.8 Thus, in this study, the CO2 absorption and desorption process of the VAm-GP solution (50 ml, 4 mg ml−1) was monitored by measuring the conductivity of the solution with a conductivity meter (SevenMulti, METTLER TOLEDO, Greifensee, Switzerland). The stable conductivity of the GP solution after bubbling N2 (10 ml min−1) at 30 °C was considered the baseline. In the CO2 absorption process, 10% CO2 (90% N2) was guided into the GP solution at 30 °C. The conductivity of the CO2 saturated solution was recorded as the conductivity of the CO2-absorbed GP solution. Then, the GP solution was moved to a 75 °C water bath to desorb CO2 while bubbling N2. After the conductivity of the solution became constant at 75 °C, the GP solution was moved to the 30 °C water bath again with N2 bubbling to exclude the effect of temperature on the conductivity. The stable conductivity under these condition was recorded as the conductivity of the CO2-desorbed GP solution. This cycle was performed twice.
Results and discussion
Polymerization of the NVF-GPs
To obtain GPs containing large amounts of VAm via the hydrolysis of NVF-GPs (Scheme 1), it was necessary to prepare NVF-GPs with a high NVF content. Table 1a shows the synthesis result when the NVF feed ratio was varied. Homogenous aqueous solutions of the GPs were obtained when the NVF feed ratio was varied from 0 to 10 mol%. However, precipitation occurred when the NVF feed ratio was increased to 20 and 30 mol%, indicating a low colloidal stability of the growing GPs. The reaction solution was gelled when 50 mol% of NVF was polymerized at 70 °C; however, precipitation was observed when it was polymerized at 90 °C (Table 1b), thus indicating that pNIPAm that is polymerized with 50 mol% NVF has a lower critical solution temperature between 70 and 90 °C.
The NVF incorporation ratio was confirmed by the 1H NMR spectra of the GPs. Figure 1 shows the 1H NMR spectra of the NVF0-BIS5, NVF5-BIS5 and NVF10-BIS5 GPs, which were polymerized with 5 mol% BIS, and 0, 5 and 10 mol% NVF, respectively. The NVF incorporation ratio was calculated from the integration of the peak at ~δ 8 p.p.m., which corresponds to the aldehyde in the NVF. The results showed that the incorporation rate of NVF was comparable to the NVF feed ratio.
The temperature-dependent change in size of the NVF0-BIS5, NVF5-BIS5 and NVF10-BIS5 GPs is shown in Figure 2. All of the GPs exhibited volume-phase transition behavior at temperatures between 30 and 50 °C. As the NVF feed ratio increased, there was an increase in both the GP size and the VPTT, possibly as a result of the increased hydrophilicity of the GPs.16 The observation was comparable to that observed for temperature-responsive linear copolymers that contain NVF.20, 21, 22, 29
To obtain stable GPs with a high NVF feed, the concentration of CTAB was increased from 2 to 10 mM and 20 mM. Here we focused on the preparation of GPs with 30 mol% NVF (not 50 mol%), because GPs must have a phase transition temperature between 35 and 70 °C to show efficient CO2 release at 75 °C.16 As shown in Table 1c, the GP (NVF30-BIS5) polymerized with 10 mM CTAB precipitated, whereas a homogeneous GP solution was obtained with 20 mM CTAB.
Our previous study has shown that the foaming of amine-functionalized GP solutions during CO2 bubbling is a severe problem that inhibits their application. Increasing the degree of crosslinking in GPs is an effective method for abating foam formation.28 Therefore, GPs with both a high incorporation rate of NVF and a high degree of crosslinking were favored in this study. Table 1d shows the results of GP polymerization with 30 mol% NVF and different amounts of BIS. GPs with good colloid stability were obtained when the feed ratio of BIS was increased from 5 to 10 mol%. However, when the BIS amount was further increased to 20 mol%, the GPs precipitated. It has been reported that GP preparation is also affected by the total monomer concentration; smaller GPs with a high stability can be obtained by decreasing the total monomer concentration.16 Hence, GPs comprising 30 mol% NVF and 20 mol% BIS were polymerized from monomer solutions of different total concentrations in which the ratio of monomer, surfactant and initiator was kept constant. As shown in Table 1e, when the total monomer concentration was halved to 156 mM, GPs with a good colloid stability and monomodal size distribution were obtained. The hydrodynamic diameter of the NVF30-BIS20 was 156 nm, and the polydispersity index was 0.2. However, GPs with a monomodal size distribution could not be prepared when the total monomer concentration was further lowered to 62.4 mM.
Elemental analysis of GP NVF30-BIS20 (entry 12 in Table 1) was conducted to estimate the amount of NVF incorporated in the GPs. The observed C/N ratio of GP NVF30-BIS20 was 3.81. Although this value was slightly higher than the theoretical value of 3.79, it can be concluded that the GP contained more than 28 mol% (2.6 mmol g−1) of NVF.
Thus, to maximize the amine feed and to avoid foaming formation, GP NVF30-BIS20, which contained 30 mol% NVF and 20 mol% BIS, was successfully obtained by increasing the concentration of CTAB while optimizing the total monomer concentration.
Hydrolysis of NVF-GPs
To prepare a CO2 absorbent, the formamide groups in the NVF30-BIS20 GPs were hydrolyzed into amine groups after the purification of the GPs by dialysis. The hydrolysis was first conducted in an aqueous solution at room temperature; however, the GPs aggregated immediately after adding acid or base, owing to the salting out effect. To prevent aggregation, the hydrolysis solvent was changed from water to methanol, which is a better solvent for pNIPAm.30 As expected, aggregation did not occur after adding acid to the methanol solution of the NVF30-BIS20 GPs. Hydrolysis was performed in the presence of different acids, as detailed in Table 2.
The degree of hydrolysis can be determined from the change in peak integration of ~δ 8 p.p.m., which corresponds to the aldehyde in the 1H NMR spectra before and after hydrolysis. Figures 3a and b show the 1H NMR spectra of the NVF0-BIS20 and NVF30-BIS20 GPs, respectively. In contrast to the 1H NMR spectrum of the NVF0-BIS20 GPs, the 1H NMR spectrum of the NVF30-BIS20 GPs showed a clear peak near δ 8 p.p.m. that corresponds to the aldehyde in the NVF containing the GPs. As shown in Figure 3c, after hydrolysis in HCl, the broad peak near δ 8 p.p.m. disappeared, and a new sharp peak appeared at δ 8.5 p.p.m., which was attributed to the proton of formic acid that was produced during hydrolysis, thus demonstrating the complete hydrolysis of the NVF-GPs. Table 2 summarizes the degree of hydrolysis in the NVF-GPs for each of the hydrolysis conditions. The aldehyde in the NVF30-BIS20 GPs was be completely hydrolyzed in the presence of strong acids such as HCl and H2SO4. The resulting GPs are noted as VAm30-BIS20.
The amount of amine in the completely hydrolyzed NVF30-BIS5 GPs was quantified via pH titration as 3.2 mmol amine per g GPs, which was approximately six times larger than that of the reported temperature-responsive p(NIPAm-co-VAm) GPs.23
The hydrodynamic diameters of the NVF30-BIS20 GPs before and after complete hydrolysis were investigated in Figure 4. The diameters of the GPs did not show any significant differences in the swollen state; however, the VPTT of the VAm30-BIS20 GPs increased from 50 to 65 °C, and the diameters of the GPs after hydrolysis (140–145 nm) were larger than they were before hydrolysis (130–135 nm) at 70–85 °C. This result indicated an increased hydrophilicity and osmotic pressure of the amine groups compared with the formamide groups. The diameters of the hydrolyzed GPs at 25 °C were slightly smaller than those at 40 °C. Although the reason for the difference is not clear, we did not investigate this observation further in this study.
The VPTT of the VAm30-BIS20 GPs, 65 °C, is between the reported ideal CO2 capture temperature (30 °C) and release temperature (75 °C).14, 15, 16 The hydrophilic–hydrophobic phase transition of the GPs is expected to improve the reversible CO2 capture capacity of the GPs, according to our previous report.16
Reversible CO2 absorption and desorption
The reversible CO2 absorption and desorption process of the VAm30-BIS20 GPs was monitored by measuring the conductivity of the GP solution during flowing of CO2/N2 at 30 °C/75 °C.
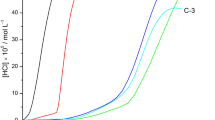
As shown in Figure 5, when CO2 was bubbled into the GP solution at 30 °C, the conductivity increased from 30 to 95 μS cm−1, thus indicating the formation of ions during CO2 absorption. Consequently, when N2 was bubbled into the solution at 75 °C, the conductivity decreased to 45 μS cm−1, which corresponded to CO2 desorption. Because conductivity depends slightly on temperature, after CO2 release at 75 °C, the GP solution was moved into a 30 °C water bath to stabilize the conductivity during N2 flow. However, the conductivity stabilized at 40 μS cm−1, and it did not return to the initial value of 30 μS cm−1, thus indicating that the CO2 was not completely released, probably because a certain amount of strong covalent bonds (carbamate) formed between the primary amine and CO2.31
By shifting the gas flow from N2 to CO2, the conductivity of the GP solution increased to 90 μS cm−1, owing to CO2 absorption. However, when the GP solution was moved to a 75 °C water bath to release CO2 with the flow of N2, the conductivity decreased to only 55 μS cm−1, and aggregation of the GPs was observed. Aggregation may possibly have been caused by inter-GP crosslinking via the electrostatic interactions between a positively charged ammonium and negatively charged carbamate, which formed between two primary amine molecules and one CO2 molecule.31 Precipitation can be prevented by replacing the primary amine with a tertiary amine or a hindered secondary amine by further modification of pVAm.14, 15, 16, 17, 31 The reversible CO2 capture efficiency may also be improved by the modification.
Importantly, foaming was not observed throughout the bubbling process of reversible CO2 capture using the solution of NVF30-BIS20 GPs. However, when we used the solution of DMAPM30-BIS5 GPs, severe foaming was observed, thus indicating the importance of optimizing the amount of crosslinking to avoid foaming of the GP solution.14
Conclusion
To maximize the amine concentration and avoid foam formation, stable thermal-responsive NVF30-BIS20 GPs containing 20 mol% of BIS crosslinker and a large amount of NVF (30 mol%) were designed and prepared by increasing the surfactant concentration and optimizing the total monomer concentration. Subsequently, VAm30-BIS20 GPs were obtained via complete hydrolysis of NVF30-BIS20 GPs in methanol in the presence of a strong acid. The amine content in the 30 mol% p(NIPAm-co-VAm) GPs in this study was as high as 3.2 mmol amine per g GPs, which is approximately six times larger than the reported value of thermal-responsive VAm GPs.
The VAm30-BIS20 GPs exhibited a VPTT of 65 °C; therefore, it reversibly absorbed and desorbed CO2 with a small temperature change from 30 to 75 °C without foam formation. In the future, we expect that the amine content in the GPs could be further increased by carefully controlling the polymerization conditions and by incorporating more hydrophobic monomers, such as t-butylpropylacrylamide, because t-butylpropylacrylamide can decrease the GP size and lower the VPTT, according to a previous study.16 The aggregation of GPs during the CO2 capture process may be prevented by converting the primary amine into a secondary or tertiary amine.

Preparation of vinylamine gel particless.
References
Solomon, S., Qin, D., Manning., M., Marquis, M., Averyt, K., Tignor, M. M. B. Jr, Miller, H. L. & Chen, Z. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, Cambridge, 2007).
Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652 (2009).
Chowdhury, F. A., Okabe, H., Yamada, H., Onoda, M. & Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 4, 201 (2011).
Matsuzaki, Y., Yamada, H., Chowdhury, F. A., Higashii, T., Kazama, S. & Onoda, M. Ab initio study of CO2 capture mechanisms in monoethanolamine aqueous solution: reaction pathways from carbamate to bicarbonate. Energy Procedia 37, 400 (2013).
Murai, S., Kato, Y., Maezawa, Y., Muramatsu, T. & Saito, S. Novel hindered amine absorbent for CO2 capture. Energy Procedia 37, 417 (2013).
Goeppert, A., Czaun, M., May, R. B., Prakash, G. K. S., Olah, G. A. & Narayanan, S. R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 133, 20164 (2011).
Keskin, S., van Heest, T. M. & Sholl, D. S. Can metal–organic framework materials play a useful role in large-scale carbon dioxide separations? ChemSusChem 3, 879 (2010).
Furusho, Y. & Endo, T. Capture and release of CO2 by polyamidine. J. Polym. Sci. A Polym. Chem. 51, 3404 (2013).
Ochiai, B., Yokota, K., Fujii, A., Nagai, D. & Endo, T. Reversible trap−release of CO2 by polymers bearing DBU and DBN moieties. Macromolecules 41, 1229 (2008).
Trewin, A. & Cooper, A. I. Porous organic polymers: distinction from disorder? Angew. Chem. Int. Ed. 49, 1533 (2010).
Lu, W., Sculley, J. P., Yuan, D., Krishna, R., Wei, Z. & Zhou, H. C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 51, 7480 (2012).
Choi, S., Drese, J. H. & Jones, C. W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796 (2009).
D'Alessandro, D. M., Smit, B. & Long, J. R. Carbon dioxide capture: prospects for new materials. Angew. Chem., Int. Ed. 49, 6058 (2010).
Hoshino, Y., Imamura, K., Yue, M., Inoue, G. & Miura, Y. Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J. Am. Chem. Soc. 134, 18177 (2012).
Yue, M., Hoshino, Y., Ohshiro, Y., Imamura, K. & Miura, Y. Temperature-responsive microgel films as reversible carbon dioxide absorbents in wet environment. Angew. Chem. Int. Ed. 53, 2654 (2014).
Yue, M., Hoshino, Y. & Miura, Y. Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles. Chem. Sci. 6, 6112 (2015).
Werz, P. D. L., Kainz, J. & Rieger, B. Thermo- and pH-responsive nanogel particles bearing secondary amine functionalities for reversible carbon dioxide capture and release. Macromolecules 48, 6433 (2015).
Pelton, R. H. & Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloid Surf. 20, 247 (1986).
Fujishig, S., Kubota, K. & Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 93, 3311 (1989).
Akashi, M., Nakano, S. & Kishida, A. Synthesis of poly(N-vinylisobutyramide) from poly(N-vinylacetamide) and its thermosensitive property. J. Polym. Sci. A Polym. Chem. 34, 301 (1996).
Tachaboonyakiat, W., Ajiro, H. & Akashi, M. Synthesis of a thermosensitive polycation by random copolymerization of N-vinylformamide and N-vinylbutyramide. Polym. J. 45, 971 (2013).
Yamamoto, K., Serizawa, T., Muraoka, Y. & Akashi, M. Synthesis and functionalities of poly(N-vinylalkylamide). 13. Synthesis and properties of thermal and pH stimuli-responsive poly(vinylamine) copolymers. Macromolecules 34, 8014 (2001).
Takemoto, Y., Ajiro, H., Asoh, T.-A. & Akashi, M. Fabrication of surface-modified hydrogels with polyion complex for controlled release. Chem. Mater. 22, 2923 (2010).
Xu, J., Timmons, A. B. & Pelton, R. H. N-Vinylformamide as a route to amine-containing latexes and microgels. Colloid. Polym. Sci. 282, 256 (2004).
Fineman, M. & Ross, S. D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 5, 259 (1950).
Kelen, T. & Tudos, F. J. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J. Macromol. Sci. Chem. 9, 1 (1975).
Thaiboonrod, S., Berkland, C., Milani, A. H., Ulijn, R. & Saunders, B. R. Poly(vinylamine) microgels: pH-responsive particles with high primary amine contents. Soft Matter 9, 3920 (2013).
Abe, S. & Yamaguchi, M. Study on the foaming of crosslinked polyethylene. J. Appl. Polym. Sci. 79, 2146 (2001).
Yue, M., Imai, K., Yamashita, C., Miura, Y. & Hoshino, Y. Effects of hydrophobic modifications and phase transitions of polyvinylamine hydrogel films on reversible CO2 capture behavior: comparison between copolymer films and blend films for temperature-responsive CO2 absorption. Macromol. Chem. Phys. 218, 1600570 (2017).
Winnik, F. M., Ringadorf, H. & Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 23, 2415 (1990).
Vaidya, P. D. & Kenig, E. Y. CO2-alkanolamine reaction kinetics: a review of recent studies. Chem. Eng. Technol. 30, 1467 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yue, M., Imai, K., Miura, Y. et al. Design and preparation of thermo-responsive vinylamine-containing micro-gel particles for reversible absorption of carbon dioxide. Polym J 49, 601–606 (2017). https://doi.org/10.1038/pj.2017.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.28
This article is cited by
-
Hydrogel particles for CO2 capture
Polymer Journal (2024)
-
Adsorption of Pb(II) ion by pyromellitic dianhydride-modified polyvinylamine under programmed-temperature
Chemical Papers (2023)
-
Frontiers of CO2 Capture and Utilization (CCU) towards Carbon Neutrality
Advances in Atmospheric Sciences (2022)
-
Synthesis and characterization of cryogels of p(HEMA-N-vinylformamide) and p(HEMA-N-Vinylpyrrolidone) for chemical release behaviour
Journal of Porous Materials (2021)
-
Preparation of palladium-loaded polymer hydrogel catalysts with high durability and recyclability
Polymer Journal (2020)