Abstract
Background:
At the population level, obesity is associated with prostate cancer (PC) mortality. However, few studies analyzed the associations between obesity and long-term PC-specific outcomes after initial treatment.
Methods:
We conducted a retrospective analysis of 4268 radical prostatectomy patients within the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cox models accounting for known risk factors were used to examine the associations between body mass index (BMI) and PC-specific mortality (PCSM; primary outcome). Secondary outcomes included biochemical recurrence (BCR) and castration-resistant PC (CRPC). BMI was used as a continuous and categorical variable (normal <25 kg/m2, overweight 25–29.9 kg/m2 and obese ⩾30 kg/m2). Median follow-up among all men who were alive at last follow-up was 6.8 years (interquartile range=3.5–11.0). During this time, 1384 men developed BCR, 117 developed CRPC and 84 died from PC. Hazard ratios were analyzed using competing-risks regression analysis accounting for non-PC death as a competing risk.
Results:
On crude analysis, higher BMI was not associated with risk of PCSM (P=0.112), BCR (0.259) and CRPC (P=0.277). However, when BMI was categorized, overweight (hazard ratio (HR) 1.99, P=0.034) and obesity (HR 1.97, P=0.048) were significantly associated with PCSM. Obesity and overweight were not associated with BCR or CRPC (all P⩾0.189). On multivariable analysis adjusting for both clinical and pathological features, results were little changed in that obesity (HR=2.05, P=0.039) and overweight (HR=1.88, P=0.061) were associated with higher risk of PCSM, but not with BCR or CRPC (all P⩾0.114) with the exception that the association for overweight was no longer statistical significant.
Conclusions:
Overweight and obesity were associated with increased risk of PCSM after radical prostatectomy. If validated in larger studies with longer follow-up, obesity may be established as a potentially modifiable risk factor for PCSM.
Similar content being viewed by others
Introduction
At the population level among cancer-free men, obesity is associated with future risk of prostate cancer (PC) mortality.1, 2, 3, 4, 5 Thus, obesity may biologically be associated with aggressive PC. However, other explanations exist. First, obese men may be less aggressively screened leading to delayed detection. Second, PC can be more difficult to detect in obese men including physical challenges in performing a digital rectal examination6 and lower PSA7, 8, 9, 10 further contributing to delayed detection. Third, obese men may receive less aggressive and less effective treatment. Four, obese men are less likely to undergo radical prostatectomy (RP), which some studies have shown results in lower PC death rates,11, 12, 13, 14 and obese men are more likely to have positive margins at surgery.15 Recent reports have confirmed that obesity is associated with high-grade PC at diagnosis.1, 16, 17 We also previously showed that obese men undergoing RP had higher-grade and larger tumors.18 How obesity impacts long-term PC outcomes among men diagnosed early with localized disease and treatment aggressively is less clear.
A meta-analysis found a 21% increased risk of biochemical recurrence (BCR) after RP per 5 kg/m2 increase in body mass index (BMI) among 16 studies.2 However, only six studies followed men after treatment for PC-specific mortality (PCSM) and found a trend (hazard ratio (HR) 1.20 per 5 kg/m2; P=0.06) for BMI to be associated with increased PCSM.2 Of these six studies, only one examined a RP population of 5 313 men and found no significant association between BMI and PCSM, although this study was single center and nearly all men were Caucasian.19 Since publication of that meta-analysis, one other study20 examined an RP cohort and found that a BMI 30–<35 kg/m2 was associated with PCSM (HR 1.51, P=0.040), whereas a BMI ⩾35 kg/m2 was not (HR 1.58, P=0.356). This study was also a single center of nearly all Caucasian men.20
Using the Shared Equal Access Regional Cancer Hospital (SEARCH) database, we previously reported that a BMI ⩾35 kg/m2 was associated with a higher BCR risk compared with normal weight,21 as shown by others.22, 23 However, BCR is not always correlated with PCSM.24, 25 Examining longer-term outcomes is needed, including response to salvage therapy (that is, androgen-deprivation therapy (ADT)) and ultimately PCSM. How obesity influences outcomes after salvage ADT is unknown except for one prior study from our group using SEARCH, which only examined men who received early hormonal therapy for BCR after RP.26
Using the SEARCH database, we examined the effect of obesity at the time of RP on long-term PC-specific outcomes after RP including BCR, castrate resistant PC (CRPC) and PCSM (our primary outcome). We hypothesized obesity is associated with worse prognosis in all outcome measures.
Materials and methods
Study population
After obtaining institutional review board approval, we combined data from patients undergoing RP at six Veterans Affairs Medical Centers (West Los Angeles, San Diego and Palo Alto, CA; Augusta, GA; and Durham and Asheville, NC) into SEARCH.27 We included men treated in 1990 or later as few men treated before that had BMI data available. We excluded patients with missing data on PSA (n=108), biopsy Gleason score (n=399), BMI (n=362), pathological Gleason score (n=34), positive surgical margins (n=38), extracapsular extension (n=90) and seminal vesicle invasion (n=19), resulting in a study population of 4268 men.
Statistical analysis
Our primary outcome was PCSM after RP. Death from PC was defined as death in any patient with metastases showing progression following ADT without another cause of death based on a thorough chart review. Secondary outcomes included BCR, CRPC and time to secondary treatments (radiation (XRT) or ADT). BCR was defined as a single PSA>0.2 ng ml−1, two concentrations at 0.2 ng ml−1 or salvage treatment for an elevated post-operative PSA.
As PCSM can result from either aggressive disease or less aggressive treatment, we also evaluated whether BMI was associated with receipt of secondary therapies such as adjuvant/salvage radiation therapy and ADT.
Patients who received radiation for an undetectable PSA were considered as having adjuvant radiation and were censored for BCR at that time as not having recurred. However, these men were included in models predicting CRPC and PCSM. CRPC was defined using the PC Working Group Two criteria: a 25% PSA increase from ADT PSA nadir and a PSA increase ⩾2 ng ml−1.28 Patients who never received ADT were included in the models and considered as not reaching the end-point of CRPC. Our exposure, BMI was abstracted from the medical records at the time of, but before RP and categorized as normal weight (<25 kg/m2), overweight (25–29.9 kg/m2) and obese (⩾30 kg/m2). Differences in demographic and clinicopathological features across BMI categories were examined using analysis of variance-tests for normally distributed continuous variables, Kruskal–Wallis tests for non-normally distributed continuous variables, and χ2 tests for categorical variables.
The associations between BMI and various end points were analyzed using crude and adjusted competing-risks regression models accounting for non-PC death as a competing risk.28 Given the modest number of PCSM and CRPC events raising concerns that fully adjusted models may be overfit, the crude analyses were considered primary and the adjusted models secondary analyses. For BCR, there were sufficient events such that overfitting is not an issue and thus the multivariable models were considered primary. Time zero for all analyses was the time of RP. BMI was treated as a continuous and categorical variable. Two adjusted models were fit. The first model was adjusted for VA center and clinical characteristics: age at surgery (continuous), PSA (log-transformed and continuous), biopsy Gleason sum (2–6 vs 7 (3+4) vs 7 (4+3) vs 8–10) and surgery year (continuous). The second model was additionally adjusted for pathological characteristics: pathological Gleason sum (2–6 vs 7 (3+4) vs 7 (4+3) vs 8–10), positive margins (no vs yes), extracapsular extension (no vs yes), seminal vesicles (no vs yes) and lymph node involvement (no/not done vs yes). Collinearity among variables was tested with the variance inflation factor and none of the covariates were collinear. Results are shown graphically using cumulative incidence curves.
SAS 9.3 (SAS Institute, Cary, NC, USA) and Stata 13.1 (Stata, College Station, TX, USA) were used and statistical significance was two-sided with P<0.05.
Results
Baseline characteristics by BMI categories
Baseline characteristics of the 4268 men are shown in Table 1. Overall, 955 (22%) men had normal weight, 1941 (45%) were overweight and 1372 (32%) were obese. A higher BMI was associated with younger age at surgery (P<0.001), lower PSA (median 7.1 vs 6.3 vs 6.1; P<0.001), and a shorter follow-up time (P=0.050). Biopsy Gleason sum (P=0.003) and pathological Gleason sum (P=0.028), were different across BMI categories, men with higher BMI had fewer Gleason 2–6 tumors (53% vs 46% vs 44%; 33% vs 31% vs 28%, respectively), There was no association with disease stage (P=0.093), positive margins (P=0.186), extracapsular extension (P=0.472), seminal vesicle invasion (P=0.647) and positive lymph nodes (P=0.480).
Median follow-up among all men who were alive at last follow-up was 6.8 years (interquartile range=3.5–11.0). Follow-up data for >10 years was available on 1309 men. During this time 1384 (32.4%) men developed BCR, 117 (2.7%) developed CRPC and 84 (2.0%) died from PC.
Primary outcome: PCSM among BMI categories
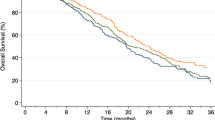
On competing risk univariable analysis, both overweight (HR=1.99, 95% confidence interval (CI) 1.06–3.76, P=0.034) and obesity (HR=1.97, 95% CI 1.01–3.86, P=0.048) were associated with increased PCSM risk (Table 2; Figure 1). Results were similar after adjusting for clinical characteristics (Table 2). Although results were slightly attenuated after further adjusting for pathological features with overweight (HR=1.88, 95% CI 0.97–3.63, P=0.061) no longer being statistically significantly associated with PCSM, the association with obesity (HR=2.05, 95% CI 1.04–4.06, P=0.039) remained significant (Table 2). When BMI was treated as a continuous variable, higher BMI was not associated with higher PCSM risk on both crude and multivariable analyses (all P⩾0.112), although when adjusting for clinical characteristics only a higher BMI was a predictor of PCSM (HR=1.05, 95% CI 1.02–1.10, P=0.013).
Secondary outcome: BCR among BMI categories
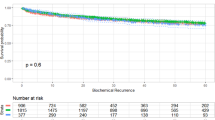
In the unadjusted model and after accounting for competing risks, BMI either as a continuous or as a categorical variable was not associated with BCR (all P⩾0.259; Table 2; Figure 2). Obesity was significantly associated with BCR after adjusting for clinical characteristics (HR=1.18, 95% CI 1.01–1.38, P=0.039) but not after adjusting for clinical and pathological features (HR=1.11, 95% CI 0.94–1.30, P=0.208; Table 2). After multivariable adjustment, overweight was not associated with BCR risk (Table 2). When BMI was treated as a continuous variable, it was not associated with BCR in univariable analysis (P=0.259) but was significantly associated with BCR after multivariable adjustment for either clinical characteristics (P=0.004) or after adjusting for clinical and pathological features (P=0.049).
Secondary outcome: secondary treatments among BMI categories
As PCSM can result from either aggressive disease or less aggressive treatment, we evaluated whether BMI was associated with receipt of secondary therapies (that is, the aggressiveness of treatment). During follow-up, 968 men received adjuvant/salvage radiation therapy and 667 received ADT.
On unadjusted analysis, obese men were equally likely to receive ADT (P=0.331; Table 3). However, after adjusting for clinical (P=0.044), but not for clinical and pathological (P=0.132) characteristics, there was a trend for obese men to be more likely to receive ADT. Overweight men had equal risks of receiving ADT (all P⩾0.273). When treated as a continuous variable, higher BMI was associated with greater risk of receiving ADT on multivariable analysis adjusted for clinical characteristics (P=0.012), although the association on unadjusted (P=0.112) or models adjusted for both clinical and pathological characteristics (P=0.077) did not reach statistical significance.
Although overweight was not associated with receipt of radiation therapy (all P⩾0.316), obese patients were ~25% more likely to receive radiation therapy compared with normal weight patients (P=0.012; Table 3). Results were similar after adjusting for clinical characteristics. Higher BMI, as a continuous variable, was associated with a higher likelihood of receiving radiation on unadjusted and adjusted analysis (all P⩽0.004).
Secondary outcome: CRPC among BMI categories
On univariable competing risk analysis, overweight and obesity were not associated with CRPC (HR 1.39, 95% CI 0.85–2.26, P=0.189; HR 1.42 95% CI 0.84–2.38, P=0.190, respectively, Table 2, Figure 3). Adjusting for clinical characteristics only (HR=1.72, 95% CI 0.99–2.98, P=0.055) or adjusting for clinical and pathological features (HR=1.56, 95% CI 0.90–2.70, P=0.114), obesity remained associated with CRPC, although the associations did not reach significance (Table 2). Although overweight maintained a similar HR for CRPC (1.34–1.36) on multivariable analyses, this was not statistically significant (P⩾0.244). As a continuous variable, higher BMI was not associated with higher risk of CRPC on both uni- and multivariable analyses (all P⩾0.064).
Discussion
Obesity is associated with aggressive PC at diagnosis.1, 16, 17 However, less is known about whether obesity has an impact on long-term PC outcomes including outcomes after primary and salvage treatments and ultimately PCSM among men diagnosed with early stage disease and treated aggressively. Previously, we found a BMI⩾35 kg/m2 was associated with BCR after RP.21 In a separate study, we found obesity was associated with higher risk of developing CRPC among a select group of men starting early ADT as salvage treatment for BCR after RP.26 We hypothesized that obesity is associated with more aggressive PC leading to worse outcomes after both primary and salvage treatments, and that obese men would be more likely to progress to CRPC and PCSM. To test this hypothesis, we analyzed the risk of long-term PC-specific outcomes in SEARCH. We found obese men had a significantly increased risk of PCSM on both unadjusted and multivariable analyses despite being more likely to receive post-operative radiation and ADT. If validated in other studies, these findings suggest higher BMI may be a modifiable risk factor for PCSM despite aggressive treatment with RP.
A systematic review and meta-analysis found a 20% (95% CI −1 to 46%) increased risk of PCSM after treatment per 5 kg/m2 increase in BMI.2 Pooled estimates were calculated from six studies of mostly Caucasian men that followed 18 203 PC patients after primary treatment. However, only one of those six studies examined a pure RP data set,19 while three studies examined PCSM after all types of treatment,4, 29, 30 one examined an external beam radiation therapy data set31 and one a brachytherapy data set.32 Nonetheless, for the BMI category ⩾30 kg/m2, the relative risk for PCSM in the individual studies was >1 in four studies (1.46–2.64) and <1 in one study (0.9; one study did not report results by categorized BMI). Another study published after the meta-analysis, which only included RP patients found higher BMI was linked with increased risk of PCSM: the HR was 1.51 for BMI 30–34.9 kg/m2 and 1.58 for BMI ⩾35 kg/m2.20 Thus, the preponderance of the literature suggests higher BMI is linked with greater PCSM after treatment with HRs between 1.5 and 2.5. However, few of the prior studies included a large percentage of black men. As such the fact that our population was all men from equal access VA Hospitals and included ~37% black is noteworthy. Moreover, only one previous study33 performed competing-risks analysis as was done in our present study. This is very important in that obesity is a well-known risk factor for non-PC death,34 including men treated with RP,20 due to competing causes of mortality (that is, obese subjects do not live long enough to have the opportunity to die from PC). Despite these key differences in our study versus prior studies, our results were similar with the prior literature (HR 1.97 for obesity in our unadjusted results) suggesting an unequivocal link between obesity and PCSM.
In secondary analysis, on multivariable analysis each BMI unit was associated with a significant, albeit modest, 1–2% increased risk of BCR among 4263 racially diverse patients (~37% black men). A systematic review and meta-analysis of 16 studies of mostly Caucasian men, which followed 26 479 PC patients after primary treatment, found a 21% (95% CI 11–31%) increased risk of BCR per 5 kg/m2 increase in BMI.2 As such, despite our results on the association between obesity and PCSM being of similar magnitude as in prior studies, the association between obesity and BCR was slightly weaker than in prior studies. In our prior study from SEARCH, the excess risk of BMI was largely limited to men with BMI ⩾35 kg/m2, a group we did not examine separately in this study due to limited number of long-term events.21 In our prior study, the multivariable risk of BMI as a continuous variable adjusted for clinical features was 1.03 (95% CI 1.00–1.06), which is similar to the 1.01 (HR 1.00–1.02) in the current study. Therefore, our data are consistent with our prior results and the preponderance of the data that link higher BMI with BCR.
On adjusted analysis, despite obese men being equally likely to receive ADT, there were no differences on CRPC risk by obesity status, although the direction of the association suggested higher risks in obese men, which did not reach statistical significance. Only one prior study examined outcomes after ADT by BMI.26 In that study, from SEARCH, we found a suggestion that higher BMI was associated with higher risk for CRPC, although that study only included men treated with early ADT. Herein, we extended our findings to all surgically treated patients again finding a non-significant suggestion that higher BMI is associated with CRPC. Moreover, as the direction of the association (higher BMI equals higher risk) is consistent with our findings that higher BMI was associated with higher risks of BCR and PCSM and as it would be unusual for a risk factor to be associated with BCR and PCSM but not CRPC, this argues that the association with CRPC is likely real, but underpowered. Nonetheless, given the non-significant nature of the results larger studies with longer follow-up are needed to confirm our findings.
One possible explanation for the worse outcomes among obese men is that operating on obese men can be technically challenging. Prior studies showed that obesity is associated with capsular incision, reflecting a less-than ideal operation.35 However, obesity remained associated with poor outcome even after adjusting for pathological features including margin status,2, 36 suggesting poor technique alone cannot explain the association between obesity and aggressive PC. Potential mechanisms that may link obesity with poor outcomes include higher serum insulin, insulin-like growth factor-1, and leptin and lower adiponectin levels in obese men.37 In addition, obese men tend to have lower serum testosterone, which some studies have linked with an increased risk of aggressive PC.38 Also, obesity is associated with excess inflammation that may promote the development of more aggressive tumors.4 Finally, in regards to outcomes after ADT, there is a suggestion that traditional ADT, which is given in fixed doses not adjusted for body surface area, results in less effective testosterone suppression in obese men.39 Given data linking better testosterone suppression with lower risks for CRPC and PCSM,40 poor androgen suppression in obese men may contribute to an already underlying more aggressive biology.
Our study was retrospective and only included men from the VA system. Whether these results apply to the general population is unknown. Height and weight were not obtained in a standardized manner and are subject to human error in measurement. However, errors in BMI measurement would tend to bias the results toward the null, not create positive associations as observed in our study. Testosterone levels were unavailable to confirm castration after ADT. We only studied men who underwent RP; whether these findings apply to men undergoing other treatments is unknown. Although we did not adjust for other treatments received between the time of BCR and PC death, we found that obese men were in general either equally or more likely to receive salvage treatments. Certainly there were no data to suggest obese men were less likely to receive salvage treatments. Thus, decreased receipt of these therapies cannot explain the association between obesity and PCSM. Also, the number of events was modest. As such, there are potential concerns that our multivariable models may be overfitted. To account for this, we focused on the crude analyses as our primary outcome for these later outcomes of PCSM and CRPC. However, the results of our multivariable models were nearly the same as the unadjusted models, minimizing concerns that our adjusted models were overfitted. Nonetheless, larger studies with longer follow-up are needed to confirm our results. Finally, our results support an association between obesity and PCSM. This does not imply obesity causes more aggressive PC. Rather, obesity may be associated with other factors such as poor diet or lack of exercise, which we could not adjust for, which may explain this association. More work is needed to understand the potential explanations for the obesity-aggressive PC link.
In summary, our study supports the hypothesis that overweight and obese men are at an increased risk of PCSM. Further studies using larger populations with longer follow-up are necessary to validate these findings, but if validated, these findings suggest BMI may be a modifiable risk factor for PCSM after RP.
References
Moller H, Roswall N, Van Hemelrijck M, Larsen SB, Cuzick J, Holmberg L et al. Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. Int J Cancer 2015; 136: 1940–1947.
Cao Y, Ma J . Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res 2011; 4: 486–501.
Haque R, Van Den Eeden SK, Wallner LP, Richert-Boe K, Kallakury B, Wang R et al. Association of body mass index and prostate cancer mortality. Obes Res Clin Pract 2014; 8: e374–e381.
Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008; 9: 1039–1047.
Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM et al. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92-02. J Clin Oncol 2008; 26: 4333–4339.
Chu DI, De Nunzio C, Gerber L, Thomas JA 2nd, Calloway EE, Albisinni S et al. Predictive value of digital rectal examination for prostate cancer detection is modified by obesity. Prostate Cancer Prostatic Dis 2011; 14: 346–353.
Banez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 2007; 298: 2275–2280.
Fowke JH, Signorello LB, Chang SS, Matthews CE, Buchowski MS, Cookson MS et al. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer 2006; 107: 2361–2367.
Grubb RL 3rd, Black A, Izmirlian G, Hickey TP, Pinsky PF, Mabie JE et al. Serum prostate-specific antigen hemodilution among obese men undergoing screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev 2009; 18: 748–751.
Barqawi AB, Golden BK, O'Donnell C, Brawer MK, Crawford ED . Observed effect of age and body mass index on total and complexed PSA: analysis from a national screening program. Urology 2005; 65: 708–712.
Lee BH, Kibel AS, Ciezki JP, Klein EA, Reddy CA, Yu C et al. Are biochemical recurrence outcomes similar after radical prostatectomy and radiation therapy? analysis of prostate cancer-specific mortality by nomogram-predicted risks of biochemical recurrence. Eur Urol 2015; 67: 204–209.
Tewari A, Johnson CC, Divine G, Crawford ED, Gamito EJ, Demers R et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol 2004; 171: 1513–1519.
Nepple KG, Stephenson AJ, Kallogjeri D, Michalski J, Grubb RL 3rd, Strope SA et al. Mortality after prostate cancer treatment with radical prostatectomy, external-beam radiation therapy, or brachytherapy in men without comorbidity. Eur Urol 2013; 64: 372–378.
Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010; 28: 1508–1513.
Jayachandran J, Aronson WJ, Terris MK, Presti JC Jr, Amling CL, Kane CJ et al. Obesity and positive surgical margins by anatomic location after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. BJU Int 2008; 102: 964–968.
Discacciati A, Orsini N, Wolk A . Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol 2012; 23: 1665–1671.
Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL Jr, Freedland SJ . Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol Biomarkers Prev 2014; 23: 2936–2942.
Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW . Obese men have higher-grade and larger tumors: an analysis of the Duke prostate center database. Prostate Cancer Prostatic Dis 2009; 12: 259–263.
Siddiqui SA, Inman BA, Sengupta S, Slezak JM, Bergstralh EJ, Leibovich BC et al. Obesity and survival after radical prostatectomy: a 10-year prospective cohort study. Cancer 2006; 107: 521–529.
Chalfin HJ, Lee SB, Jeong BC, Freedland SJ, Alai H, Feng Z et al. Obesity and long-term survival after radical prostatectomy. J Urol 2014; 192: 1100–1104.
Freedland SJ, Aronson WJ, Kane CJ, Presti JC Jr, Amling CL, Elashoff D et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol 2004; 22: 446–453.
Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology 2005; 66: 1060–1065.
Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 2004; 22: 439–445.
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439.
Jhaveri FM, Zippe CD, Klein EA, Kupelian PA . Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology 1999; 54: 884–890.
Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int 2012; 110: 492–498.
Allott EH, Abern MR, Gerber L, Keto CJ, Aronson WJ, Terris MK et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis 2013; 16: 391–397.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR . Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer 2007; 109: 1192–1202.
Davies BJ, Smaldone MC, Sadetsky N, Dall’era M, Carroll PR . The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol 2009; 182: 112–117.
Efstathiou JA, Chen MH, Renshaw AA, Loffredo MJ, D’Amico AV . Influence of body mass index on prostate-specific antigen failure after androgen suppression and radiation therapy for localized prostate cancer. Cancer 2007; 109: 1493–1498.
van Roermund JG, Hinnen KA, Battermann JJ, Witjes JA, Bosch JL, Kiemeney LA et al. Body mass index is not a prognostic marker for prostate-specific antigen failure and survival in Dutch men treated with brachytherapy. BJU Int 2010; 105: 42–48.
Masters RK, Powers DA, Link BG . Obesity and US mortality risk over the adult life course. Am J Epidemiol 2013; 177: 431–442.
Freedland SJ, Grubb KA, Yiu SK, Nielsen ME, Mangold LA, Isaacs WB et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol 2005; 174: 1798–1801.
Okotie OT, Aronson WJ, Wieder JA, Liao Y, Dorey F, DeKernion JB et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol 2004; 171: 2260–2264.
Mistry T, Digby JE, Desai KM, Randeva HS . Obesity and prostate cancer: a role for adipokines. Eur Urol 2007; 52: 46–53.
Allott EH, Masko EM, Freedland SJ . Obesity and prostate cancer: weighing the evidence. Eur Urol 2013; 63: 800–809.
Smith MR . Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res 2007; 13: 241–245.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272–277.
Acknowledgements
This paper was supported by grants NIH CA160653 to SJF and NIH/NCI P50CA09231 to WJA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Vidal, A., Howard, L., Sun, S. et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis 20, 72–78 (2017). https://doi.org/10.1038/pcan.2016.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.47
This article is cited by
-
Body mass index, triglyceride-glucose index, and prostate cancer death: a mediation analysis in eight European cohorts
British Journal of Cancer (2024)
-
Obesity and prostate cancer — microenvironmental roles of adipose tissue
Nature Reviews Urology (2023)
-
Are associations between obesity and prostate cancer outcomes following radical prostatectomy the same in smokers and non-smokers? Results from the SEARCH Cohort
Cancer Causes & Control (2023)
-
A Problem in NIH and Federally Funded Prostate Cancer Interventional Clinical Trials
Journal of Racial and Ethnic Health Disparities (2023)
-
Post-diagnostic health behaviour scores in relation to fatal prostate cancer
British Journal of Cancer (2022)