Abstract
Background:
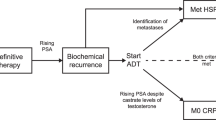
Bicalutamide is a widely used, relatively non-toxic anti-androgen, particularly when used in combination with androgen deprivation. In men on combined androgen blockade (CAB), the typical dose is 50 mg per day. For men receiving monotherapy with bicalutamide anti-androgen, the dose is 150 mg per day. The objective was to determine the PSA response rate to increasing bicalutamide to 150 mg per day in men who develop castrate-resistant prostate cancer (CRPC) on CAB with goserelin acetate and bicalutamide 50 mg per day.
Methods:
A national, multicentre, phase 2, open-label study in men on CAB with a rising PSA>2.0. The primary end point of the trial was PSA response at 12 months, defined as a decline by 50% or more compared with baseline value. Partial response was defined as a PSA decline of 10–49%. Secondary end points were duration of PSA response, change in slope of serum PSA, change in ratio of free PSA: total PSA at 3 months, 6 months and 12 months as compared with baseline; duration of the bicalutamide withdrawal response after discontinuation; the rate of cardiovascular events; and toxicity. The study was initially planned to accrue 100 patients, but was closed early due to diminishing accrual.
Results:
Sixty-four patients were accrued; 61 patients received trial treatment and constituted the intention-to-treat (ITT) cohort. 70% were M0. Among 59 evaluable ITT patients, 13 (22%) patients had a >50% PSA decline, 5 (8%) had a decline between 10 and 50%, 4 (7%) had stabilization and 37 (63%) had PSA progression. The median duration was 3.7 months (95% confidence interval of 0.92–6.21 months).
Conclusion:
In patients with early biochemical failure on CAB with bicalutamide 50 mg, an increase in dose to 150 mg of bicalutamide resulted in a PSA response of ⩾50% in 22% of patients. Toxicity was mild. Bicalutamide dose intensification may benefit a subset of patients with CRPC. We believe this relatively inexpensive approach warrants further evaluation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Huggins C, Hodges CV . Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941; 1: 293–297.
Klotz L . Maximal androgen blockade for advanced prostate cancer. Best Pract Res Clin Endocrinol Metab 2008; 22: 331–340.
Akaza H . CAB for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci 2011; 102: 51–56.
Chodak G, Gomella L . Phung de H. CAB in advanced prostate cancer: looking back to move forward. Clin Genitourin Cancer 2007; 5: 371–378.
Labrie F, Dupont A, Belanger A, Cusan L, Lacourciere Y, Monfette G et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an anti-androgen. Clin Invest Med 1982; 5: 267–275.
Labrie F, Belanger A, Simard J, Labrie C, Dupont A . Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first-line therapy. Cancer 1993; 71 (3 Suppl): 1059–1067.
Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 2000; 355: 1491.
Klotz L, Schellhammer P. CAB . the case for bicalutamide. Clin Prostate Cancer 2005; 3: 215–219.
Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S et al. Study Group for the CAB Therapy of Prostate Cancer. CAB with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009; 115: 3437–3445.
Malkowicz SB . The role of diethylstilbestrol in the treatment of prostate cancer. Urology 2001; 58 (2, Suppl. 1): 108–113.
Bergan RC, Reed E, Myers CE, Headlee D, Brawley O, Cho HK et al. A phase II study of high-dose tamoxifen in patients with hormone-refractory prostate cancer. Clin Cancer Res 1999; 5: 2366–2373.
Dawson NA, Conaway M, Halabi S, Winer EP, Small EJ, Lake D et al. A randomized study comparing standard versus moderately high dose megestrol acetate for patients with advanced prostate carcinoma: cancer and leukemia group B study 9181. Cancer 2000; 88: 825–834.
Chang AY, Bennett JM, Pandya KJ, Asbury R, McCune C . A study of aminoglutethimide and hydrocortisone in patients with advanced and refractory prostate carcinoma. Am J Clin Oncol 1989; 12: 358–360.
Samojlik E, Lippman AJ, Kirschner MA, Ertel NH, Park Y, Szmal E . Medical adrenalectomy for advanced prostatic cancer: clinical and hormonal effects. Am J Clin Oncol 1988; 11: 579–585.
Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia group B 9182 study. J Clin Oncol 1999; 17: 2506–2513.
Sartor O, Weinberger M, Moore A, Li A, Figg WD . Effect of prednisone on prostate-specific antigen in patients with hormone-refractory prostate cancer. Urology 1998; 52: 252–256.
Nishiyama T, Terunuma M . Hormone/antihormone withdrawal and dexamethasone for hormone-refractory prostate cancer. Int J Urol 1998; 5: 44–47.
Storlie JA, Buckner JC, Wiseman GA, Burch PA, Hartmann LC, Richardson RL . Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone refractory metastatic prostate carcinoma. Cancer 1995; 76: 96–100.
Nishimura K, Nonomura N, Yasunaga Y, Takaha N, Inoue H, Sugao H et al. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer 2000; 89: 2570–2576.
Small EJ, Vogelzang NJ . Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol 1997; 15: 382–388.
Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 1997; 15: 2928–2938.
Sinibaldi V, Carducci M, Abeloff M, Eisenberger M . Experience with casodex in patients in patients with stage D3 prostate cancer. Proc Am Soc Clin Oncol 1997; 16: 313a.
Joyce R, Fenton MA, Rode P, Constantine M, Gaynes L, Kolvenbag G et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol 1998; 159: 149–153.
Blackledge GR, Lowery K . Role of prostate-specific antigen as a predictor of outcome in prostate cancer. Prostate Suppl 1994; 5: 34–38.
Hu X, Lazar MA . Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 2000; 11: 6–10.
Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004; 10: 33–39.
Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL . Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res 2005; 11: 4653–4657.
Mohler JL . Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol 2008; 617: 223–234.
Mizokami A, Koh E, Fujita H, Maeda Y, Egawa M, Koshida K et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res 2004; 64: 765–771.
Kolvenbag GJ, Nash A . Bicalutamide dosages used in the treatment of prostate cancer. Prostate 1999; 39: 47–53.
Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T et al. A randomised comparison of ‘Casodex’ 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol 1998; 33: 447–456.
Wadhwa VK, Weston R, Parr NJ . Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health-related quality of life benefits for osteoporotic men with prostate cancer. BJU Int 2011; 107: 1923–1929.
McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP . Casodex Early Prostate Cancer Trialists’ Group. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 2006; 97: 247–254.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur Urol 2011; 59: 572–583.
Moinpour M, Fisher E, Moinpour CM, Coleman D, Hussain MH, Sartor AO et al. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy faileda: Southwest Oncology Group study (SWOG 9235). Urology 58: 53–58.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This study was funded by an unrestricted grant from AstraZeneca Canada. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Klotz, L., Drachenberg, D., Singal, R. et al. An open-label, phase 2 trial of bicalutamide dose escalation from 50 mg to 150 mg in men with CAB and castration resistance. A Canadian Urology Research Consortium Study. Prostate Cancer Prostatic Dis 17, 320–324 (2014). https://doi.org/10.1038/pcan.2014.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2014.24
This article is cited by
-
Abiraterone acetate versus bicalutamide in combination with gonadotropin releasing hormone antagonist therapy for high risk metastatic hormone sensitive prostate cancer
Scientific Reports (2021)
-
Timing of androgen deprivation monotherapy and combined treatments in castration-sensitive and castration-resistant prostate cancer: a narrative review
World Journal of Urology (2020)
-
Treating metastatic prostate cancer with microRNA-145
Apoptosis (2018)
-
Bicalutamide dose increase in castration-resistant disease
Nature Reviews Urology (2015)
-
Bicalutamide 150 mg as secondary hormonal therapy for castration-resistant prostate cancer
International Urology and Nephrology (2015)