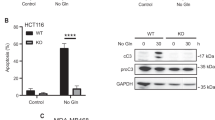
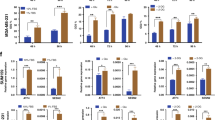
Abstract
Cancer cells depend on glutamine to sustain their increased proliferation and manage oxidative stress, yet glutamine is often depleted at tumor sites owing to excessive cellular consumption and poor vascularization. We have previously reported that p53 protein, although a well-known tumor suppressor, can contribute to cancer cell survival and adaptation to low-glutamine conditions. However, the TP53 gene is frequently mutated in tumors, and the role of mutant p53 (mutp53) in response to metabolic stress remains unclear. Here, we demonstrate that tumor-associated mutp53 promotes cancer cell survival upon glutamine deprivation both in vitro and in vivo. Interestingly, cancer cells expressing mutp53 proteins are more resistant to glutamine deprivation than cells with wild-type p53. Depletion of endogenous mutp53 protein in human lymphoma cells leads to cell sensitivity to glutamine withdrawal, whereas expression of mutp53 in p53 null cells results in resistance to glutamine deprivation. Furthermore, we found that mutp53 proteins hyper-transactivate p53-target gene CDKN1A upon glutamine deprivation, thus triggering cell cycle arrest and promoting cell survival. Together, our results reveal an unidentified mechanism by which mutp53 confers oncogenic functions by promoting cancer cell adaptation to metabolic stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB . The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20.
Young VR, Ajami AM . Glutamine: the emperor or his clothes? J Nutr 2001; 131: 2449S–2459SS.
Wise DR, Thompson CB . Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010; 35: 427–433.
Mates JM, Perez-Gomez C, Nunez de Castro I, Asenjo M, Marquez J . Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol 2002; 34: 439–458.
Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 2012; 15: 110–121.
Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010; 18: 207–219.
Roberts E, Caldwell AL, Clowes GHA, Suntzeff V, Carruthers C, Cowdry EV et al. Amino acids in epidermal carcinogenesis in mice. Cancer Res 1949; 9: 350–353.
Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 2015; 75: 544–553.
Vousden KH, Lu X . Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594–604.
Maddocks OD, Vousden KH . Metabolic regulation by p53. J Mol Med 2011; 89: 237–245.
Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013; 493: 542–546.
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005; 18: 283–293.
Reid MA, Wang WI, Rosales KR, Welliver MX, Pan M, Kong M . The B55alpha subunit of PP2A drives a p53-dependent metabolic adaptation to glutamine deprivation. Mol Cell 2013; 50: 200–211.
Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol 2011; 13: 1272–1279.
Assaily W, Rubinger DA, Wheaton K, Lin Y, Ma W, Xuan W et al. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol Cell 2011; 44: 491–501.
Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z . Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA 2010; 107: 7455–7460.
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107–120.
Budanov AV . The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell Biochem 2014; 85: 337–358.
Muller PA, Vousden KH . p53 mutations in cancer. Nat Cell Biol 2013; 15: 2–8.
Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA 1991; 88: 5413–5417.
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C et al. Mutational landscape and significance across 12 major cancer types. Nature 2013; 502: 333–339.
Zheng T, Wang J, Zhao Y, Zhang C, Lin M, Wang X et al. Spliced MDM2 isoforms promote mutant p53 accumulation and gain-of-function in tumorigenesis. Nat Commun 2013; 4: 2996.
Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139: 1327–1341.
Thangavelu K, Chong QY, Low BC, Sivaraman J . Structural basis for the active site inhibition mechanism of human kidney-type glutaminase (KGA). Sci Rep 2014; 4: 3827.
Robinson MM, McBryant SJ, Tsukamoto T, Rojas C, Ferraris DV, Hamilton SK et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem J 2007; 406: 407–414.
Campomenosi P, Monti P, Aprile A, Abbondandolo A, Frebourg T, Gold B et al. p53 mutants can often transactivate promoters containing a p21 but not Bax or PIG3 responsive elements. Oncogene 2001; 20: 3573–3579.
Friedlander P, Haupt Y, Prives C, Oren M . A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol Cell Biol 1996; 16: 4961–4971.
Ryan KM, Vousden KH . Characterization of structural p53 mutants which show selective defects in apoptosis but not cell cycle arrest. Mol Cell Biol 1998; 18: 3692–3698.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805–816.
Waldman T, Kinzler KW, Vogelstein B . p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res 1995; 55: 5187–5190.
Muller PA, Vousden KH . Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 2014; 25: 304–317.
Strano S, Dell'Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G . Mutant p53: an oncogenic transcription factor. Oncogene 2007; 26: 2212–2219.
Resnick MA, Inga A . Functional mutants of the sequence-specific transcription factor p53 and implications for master genes of diversity. Proc Natl Acad Sci USA 2003; 100: 9934–9939.
Sen N, Satija YK, Das S . PGC-1alpha, a key modulator of p53, promotes cell survival upon metabolic stress. Mol Cell 2011; 44: 621–634.
Zhou G, Wang J, Zhao M, Xie TX, Tanaka N, Sano D et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol Cell 2014; 54: 960–974.
Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun 2013; 4: 2935.
Korangath P, Teo WW, Sadik H, Han L, Mori N, Huijts CM et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin Cancer Res 2015; 21: 3263–3273.
Chakrabarti G, Moore ZR, Luo X, Ilcheva M, Ali A, Padanad M et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by β-lapachone. Cancer Metab 2015; 3: 12.
Lukey MJ, Wilson KF, Cerione RA . Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med Chem 2013; 5: 1685–1700.
Xiang Y, Stine ZE, Xia J, Lu Y, O'Connor RS, Altman BJ et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest 2015; 125: 2293–2306.
Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 2014; 13: 890–901.
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998; 282: 1497–1501.
Rosales KR, Reid MA, Yang Y, Tran TQ, Wang WI, Lowman X et al. TIPRL inhibits protein phosphatase 4 activity and promotes H2AX phosphorylation in the DNA damage response. PLoS One 2015; 10: e0145938.
Hernandez-Davies JE, Tran TQ, Reid MA, Rosales KR, Lowman XH, Pan M et al. Vemurafenib resistance reprograms melanoma cells towards glutamine dependence. J Transl Med 2015; 13: 210.
Acknowledgements
We thank members of the Kong laboratory for helpful comments on the manuscript. We also thank Dr Zhaohui Feng (Rutgers University, New Jersey) for the mutp53 constructs. This work was supported by National Institutes of Health (NIH)/R01CA183989 to MK and the V Foundation for Cancer Research to MK. MK is the Pew Scholar in Biomedical Sciences and American Cancer Society Research Scholar (RSG-16-085-01-TBE). Research reported here includes work carried out in Core Facilities, supported by the NIH/NCI under grant number P30CA33572.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tran, T., Lowman, X., Reid, M. et al. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene 36, 1991–2001 (2017). https://doi.org/10.1038/onc.2016.360
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.360
This article is cited by
-
Glutamine addiction in tumor cell: oncogene regulation and clinical treatment
Cell Communication and Signaling (2024)
-
Targeting glutamine metabolism as a therapeutic strategy for cancer
Experimental & Molecular Medicine (2023)
-
Transition of amyloid/mutant p53 from tumor suppressor to an oncogene and therapeutic approaches to ameliorate metastasis and cancer stemness
Cancer Cell International (2022)
-
Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment
Cell Communication and Signaling (2022)
-
p53-mediated redox control promotes liver regeneration and maintains liver function in response to CCl4
Cell Death & Differentiation (2022)