Abstract
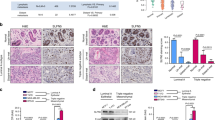
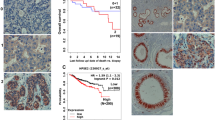
Nuclear LASP-1 (LIM and SH3 protein-1) has a direct correlation with overall survival of breast cancer patients. In this study, immunohistochemical analysis of a human breast TMA showed that LASP-1 is absent in normal human breast epithelium but the expression increases with malignancy and is highly nuclear in aggressive breast cancer. We investigated whether the chemokines and growth factors present in the tumor microenvironment could trigger nuclear translocation of LASP-1.Treatment of human breast cancer cells with CXCL12, EGF and HRG, and HMEC-CXCR2 cells with CXCL8 facilitated nuclear shuttling of LASP-1. Data from the biochemical analysis of the nuclear and cytosolic fractions further confirmed the nuclear translocation of LASP-1 upon chemokine and growth factor treatment. CXCL12-dependent nuclear import of LASP-1 could be blocked by CXCR4 antagonist, AMD-3100. Knock down of LASP-1 resulted in alterations in gene expression leading to an increased level of cell-junction and extracellular matrix proteins and an altered cytokine secretory profile. Three-dimensional cultures of human breast cancer cells on Matrigel revealed an altered colony growth, morphology and arborization pattern in LASP-1 knockdown cells. Functional analysis of the LASP-1 knockdown cells revealed increased adhesion to collagen IV and decreased invasion through the Matrigel. Proteomic analysis of immunoprecipitates of LASP-1 and subsequent validation approaches revealed that LASP-1 associated with the epigenetic machinery especially UHRF1, DNMT1, G9a and the transcription factor Snail1. Interestingly, LASP-1 associated with UHRF1, G9a, Snail1 and di- and tri-methylated histoneH3 in a CXCL12-dependent manner based on immunoprecipitation and proximity ligation assays. LASP-1 also directly bound to Snail1 which may stabilize Snail1. Thus, nuclear LASP-1 appears to functionally serve as a hub for the epigenetic machinery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Orth MF, Cazes A, Butt E, Grunewald TG . An update on the LIM and SH3 domain protein 1 (LASP1): a versatile structural, signaling, and biomarker protein. Oncotarget 2015; 6: 26–42.
Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio MC, Yates JR 3rd et al. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J Cell Biol 2004; 165: 421–432.
Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M et al. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res 2006; 312: 974–982.
Raman D, Sai J, Neel NF, Chew CS, Richmond A . LIM and SH3 protein-1 modulates CXCR2-mediated cell migration. PloS one 2010; 5: e10050.
Raman D, Neel N F, Sai J, Mernaugh RL, Ham AL, Richmond A . Characterization of chemokine receptor interacting proteins using a proteomics approach to define the CXCR2 "chemosynapse". Meth Enzymol 2009; 460: 297–312.
Neel NF . Regulation of CXC chemokine receptor function through intracellular trafficking and novel receptor-interacting proteins. PhD Dissertation, Vanderbilt University 2008.
Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA . Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer 2006; 42: 768–778.
Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 2000; 165: 5269–5277.
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008; 13: 23–35.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56.
Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004; 64: 8604–8612.
Luker KE, Luker GD . Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett 2006; 238: 30–41.
Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A . Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res 2006; 66: 5665–5675.
Li H, Yang L, Fu H, Yan J, Wang Y, Guo H et al. Association between Galphai2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 2013; 4: 1706.
Fulton AM . The chemokine receptors CXCR4 and CXCR3 in cancer. Curr Oncol Rep 2009; 11: 125–131.
Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP et al. The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunol Lett 2004; 92: 171–178.
Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 2006; 66: 7701–7707.
Grunewald TG, Kammerer U, Kapp M, Eck M, Dietl J, Butt E et al. Nuclear localization and cytosolic overexpression of LASP-1 correlates with tumor size and nodal-positivity of human breast carcinoma. BMC cancer 2007; 7: 198.
Frietsch JJ, Grunewald TG, Jasper S, Kammerer U, Herterich S, Kapp M et al. Nuclear localisation of LASP-1 correlates with poor long-term survival in female breast cancer. Br J Cancer 2010; 102: 1645–1653.
Stefansson OA, Esteller M . Epigenetic modifications in breast cancer and their role in personalized medicine. Am J Pathol 2013; 183: 1052–1063.
Zheng H, Kang Y . Multilayer control of the EMT master regulators. Oncogene 2014; 33: 1755–1763.
Robert G, Gaggioli C, Bailet O, Chavey C, Abbe P, Aberdam E et al. SPARC represses E-cadherin and induces mesenchymal transition during melanoma development. Cancer Res 2006; 66: 7516–7523.
Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol 2002; 159: 465–476.
Davis MA, Ireton RC, Reynolds AB . A core function for p120-catenin in cadherin turnover. J Cell Biol 2003; 163: 525–534.
Cipriano R, Bryson BL, Miskimen KL, Bartel CA, Hernandez-Sanchez W, Bruntz RC et al. Hyperactivation of EGFR and downstream effector phospholipase D1 by oncogenic FAM83B. Oncogene 2014; 33: 3298–3306.
Cipriano R, Miskimen KL, Bryson BL, Foy CR, Bartel CA, Jackson MW . FAM83B-mediated activation of PI3K/AKT and MAPK signaling cooperates to promote epithelial cell transformation and resistance to targeted therapies. Oncotarget 2013; 4: 729–738.
Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z . GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol 2013; 15: 201–213.
Wikman H, Westphal L, Schmid F, Pollari S, Kropidlowski J, Sielaff-Frimpong B et al. Loss of CADM1 expression is associated with poor prognosis and brain metastasis in breast cancer patients. Oncotarget 2014; 5: 3076–3087.
Mihlan S, Reiss C, Thalheimer P, Herterich S, Gaetzner S, Kremerskothen J et al. Nuclear import of LASP-1 is regulated by phosphorylation and dynamic protein-protein interactions. Oncogene 2012; 32: 2107–2113.
Li X, Meng Q, Rosen EM, Fan S . UHRF1 confers radioresistance to human breast cancer cells. Int J Radiat Biol 2011; 87: 263–273.
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 2012; 122: 1469–1486.
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer cell 2013; 23: 316–331.
Esteve PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR et al. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev 2006; 20: 3089–3103.
Peinado H, Ballestar E, Esteller M, Cano A . Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 2004; 24: 306–319.
Olmeda D, Jorda M, Peinado H, Fabra A, Cano A . Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007; 26: 1862–1874.
Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A . SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res 2007; 67: 11721–11731.
Peinado H, Olmeda D, Cano A . Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–428.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84–89.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Acknowledgements
This work was supported by the Department of Defense, IDEA grant W81XWH-11-1-0413 (to DR), VICTR award, supported in part by Vanderbilt Clinical and Translational Science Award (CTSA) grant, UL1 TR000445 from NCRR/NIH (to DR), NIH grants CA-34590 (to AR), VA Merit Award (to AR), Career Scientist Award (to AR) from the Tennessee Valley Healthcare System and the Department of Veterans Affairs and Ingram Professorship (to AR).
Author Contributions
ND-N and AK: acquisition and analysis of the data; AR: interpretation of the data and manuscript preparation; DR: conception, design, execution, acquisition, analysis of the data and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Duvall-Noelle, N., Karwandyar, A., Richmond, A. et al. LASP-1: a nuclear hub for the UHRF1-DNMT1-G9a-Snail1 complex. Oncogene 35, 1122–1133 (2016). https://doi.org/10.1038/onc.2015.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.166
This article is cited by
-
TRIM15 forms a regulatory loop with the AKT/FOXO1 axis and LASP1 to modulate the sensitivity of HCC cells to TKIs
Cell Death & Disease (2023)
-
Knockdown of long non-coding RNA LINC01123 plays a molecular sponge on miR-625-5p to inhibit the process of colorectal cancer cells via LASP1
Journal of Molecular Histology (2023)
-
LIM and SH3 protein 1 (LASP1) differentiates malignant chordomas from less malignant chondrosarcomas
Journal of Neuro-Oncology (2022)
-
Comprehensive analysis of regulation of DNA methyltransferase isoforms in human breast tumors
Journal of Cancer Research and Clinical Oncology (2021)
-
LASP1 interacts with N-WASP to activate the Arp2/3 complex and facilitate colorectal cancer metastasis by increasing tumour budding and worsening the pattern of invasion
Oncogene (2020)