Abstract
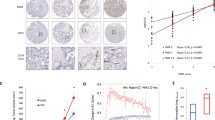
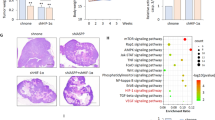
We showed previously that factor-inhibiting hypoxia-inducible factor HIF (FIH) monitors the expression of a spectrum of genes that are dictated by the cell's partial oxygen pressure. This action is mediated by the C-TAD, one of two transactivation domains (TADs) of the hypoxia-inducible factor. Here, we questioned: (1) the function of FIH as a HIF-1 modulator of gene expression in the context of a physiological oxygen gradient occurring in three-dimensional cultures and in tumors and (2) the role of FIH as a modulator of the growth of human tumor cells. We first showed that the expression pattern of HIF target genes that depend on the C-TAD, such as carbonic anhydrase IX, was spacially displaced to more oxygenated areas when FIH was silenced, whereas overexpression of FIH restricted this pattern to more hypoxic areas. Second, we showed that silencing fih severely reduced in vitro cell proliferation and in vivo tumor growth of LS174 colon adenocarcinoma and A375 melanoma cells. Finally, silencing of fih significantly increased both the total and phosphorylated forms of the tumor suppressor p53, leading to an increase in its direct target, the cell cycle inhibitor p21. Moreover, p53-deficient or mutant cells were totally insensitive to FIH expression. Thus, FIH activity is essential for tumor growth through the suppression of the p53–p21 axis, the major barrier that prevents cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- BNIP3:
-
Bcl-2/E1B 19-kDa-interacting protein 3
- CAIX:
-
carbonic anhydrase IX
- FIH:
-
factor-inhibiting HIF-1
- HIF-1α:
-
hypoxia-inducible factor-1α
References
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J . (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090.
Brady CA, Attardi LD . (2010). p53 at a glance. J Cell Sci 123: 2527–2532.
Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM et al. (2009). Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69: 358–368.
Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML et al. (2006). Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc Natl Acad Sci USA 103: 14767–14772.
Cockman ME, Webb JD, Ratcliffe PJ . (2009). FIH-dependent asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Ann NY Acad Sci 1177: 9–18.
Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J . (2008). A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron 1: 53–68.
Dayan F, Monticelli M, Pouyssegur J, Pecou E . (2009). Gene regulation in response to graded hypoxia: the non-redundant roles of the oxygen sensors PHD and FIH in the HIF pathway. J Theor Biol 259: 304–316.
Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM . (2006). The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1alpha. Cancer Res 66: 3688–3698.
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y . (1997). A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 94: 4273–4278.
Fels DR, Koumenis C . (2005). HIF-1alpha and p53: the ODD couple? Trends Biochem Sci 30: 426–429.
Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W . (1997). HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev 63: 51–60.
Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M et al. (1997). Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem 272: 8581–8593.
Hu CJ, Sataur A, Wang L, Chen H, Simon MC . (2007). The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 18: 4528–4542.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472.
Kaluz S, Kaluzova M, Stanbridge EJ . (2008). Comment on the role of FIH in the inhibitory effect of bortezomib on hypoxia-inducible factor-1. Blood 111: 5258–5259.
Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J . (2003). Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem 279: 9899–9904.
Kroeze SG, Vermaat JS, van Brussel A, van Melick HH, Voest EE, Jonges TG et al. (2010). Expression of nuclear FIH independently predicts overall survival of clear cell renal cell carcinoma patients. Eur J Cancer 46: 3375–3382.
Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK . (2002a). FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471.
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML . (2002b). Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295: 858–861.
Linke S, Stojkoski C, Kewley RJ, Booker GW, Whitelaw ML, Peet DJ . (2004). Substrate requirements of the oxygen-sensing asparaginyl hydroxylase factor inhibiting HIF. J Biol Chem 279: 14391–14397.
Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY, Wu KJ, Chiou SH, Lin SC, Chang KW . (2010). miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res 70: 1635–1644.
Lisy K, Peet DJ . (2008). Turn me on: regulating HIF transcriptional activity. Cell Death Differ 15: 642–649.
Mahon PC, Hirota K, Semenza GL . (2001). FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686.
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ et al. (2005). Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669.
Morris MR, Maina E, Morgan NV, Gentle D, Astuti D, Moch H et al. (2004). Molecular genetic analysis of FIH-1, FH, and SDHB candidate tumour suppressor genes in renal cell carcinoma. J Clin Pathol 57: 706–711.
Newbold RF . (2002). The significance of telomerase activation and cellular immortalization in human cancer. Mutagenesis 17: 539–550.
O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW . (1999). Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem 274: 2060–2071.
Pouyssegur J, Dayan F, Mazure NM . (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441: 437–443.
Sakamoto T, Seiki M . (2009). Mint3 enhances the activity of hypoxia-inducible factor-1 (HIF-1) in macrophages by suppressing the activity of factor inhibiting HIF-1. J Biol Chem 284: 30350–30359.
Schodel J, Bohr D, Klanke B, Schley G, Schlotzer-Schrehardt U, Warnecke C et al. (2010). Factor inhibiting HIF limits the expression of hypoxia-inducible genes in podocytes and distal tubular cells. Kidney Int 78: 857–867.
Semenza GL . (2010). HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20: 51–56.
Shin DH, Chun YS, Lee DS, Huang LE, Park JW . (2008). Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood 111: 3131–3136.
Tian H, McKnight SL, Russell DW . (1997). Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82.
Tian YM, Yeoh KK, Eriksson T, Kessler BM, Kramer HB, Edelmann MJ et al. (2011). Differential sensitivity of hypoxia factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J Biol Chem 286: 13041–13051.
van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D et al. (2003). Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615.
Vousden KH, Prives C . (2009). Blinded by the light: the growing complexity of p53. Cell 137: 413–431.
Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL et al. (1998). Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 92: 2260–2268.
Wiznerowicz M, Trono D . (2003). Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 77: 8957–8961.
Yan J, Jiang J, Lim CA, Wu Q, Ng HH, Chin KC . (2007). BLIMP1 regulates cell growth through repression of p53 transcription. Proc Natl Acad Sci USA 104: 1841–1846.
Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D et al. (2010). The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab 11: 364–378.
Acknowledgements
The laboratory is funded by the Ligue Nationale Contre le Cancer (équipe labellisée), the Association pour la Recherche contre le Cancer, the Institut National du Cancer, the Agence Nationale pour la Recherche, METOXIA (EU program FP7), the Centre A Lacassagne, the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale and the University of Nice (http://www.unice.fr/isdbc/). We thank Dr Corinne Bertolotto and Dr Frédéric Bost for their helpful advices. We also thank Dr M Christiane Brahimi-Horn for critical reading and editorial correction.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Pelletier, J., Dayan, F., Durivault, J. et al. The asparaginyl hydroxylase factor-inhibiting HIF is essential for tumor growth through suppression of the p53–p21 axis. Oncogene 31, 2989–3001 (2012). https://doi.org/10.1038/onc.2011.471
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.471
Keywords
This article is cited by
-
Hypermethylated gene ANKDD1A is a candidate tumor suppressor that interacts with FIH1 and decreases HIF1α stability to inhibit cell autophagy in the glioblastoma multiforme hypoxia microenvironment
Oncogene (2019)
-
MicroRNA-296: a promising target in the pathogenesis of atherosclerosis?
Molecular Medicine (2018)
-
p38α MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine
Journal of Experimental & Clinical Cancer Research (2014)
-
miR-31 is consistently inactivated in EBV-associated nasopharyngeal carcinoma and contributes to its tumorigenesis
Molecular Cancer (2014)
-
Cell cycle progression in response to oxygen levels
Cellular and Molecular Life Sciences (2014)