Abstract
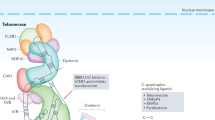
Some immortalized mammalian cell lines and tumors maintain or increase the overall length of their telomeres in the absence of telomerase activity by one or more mechanisms referred to as alternative lengthening of telomeres (ALT). Characteristics of human ALT cells include great heterogeneity of telomere size (ranging from undetectable to abnormally long) within individual cells, and ALT-associated PML bodies (APBs) that contain extrachromosomal telomeric DNA, telomere-specific binding proteins, and proteins involved in DNA recombination and replication. Activation of ALT during immortalization involves recessive mutations in genes that are as yet unidentified. Repressors of ALT activity are present in normal cells and some telomerase-positive cells. Telomere length dynamics in ALT cells suggest a recombinational mechanism. Inter-telomeric copying occurs, consistent with a mechanism in which single-stranded DNA at one telomere terminus invades another telomere and uses it as a copy template resulting in net increase in telomeric sequence. It is possible that t-loops, linear and/or circular extrachromosomal telomeric DNA, and the proteins found in APBs, may be involved in the mechanism. ALT and telomerase activity can co-exist within cultured cells, and within tumors. The existence of ALT adds some complexity to proposed uses of telomere-related parameters in cancer diagnosis and prognosis, and poses challenges for the design of anticancer therapeutics designed to inhibit telomere maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- ALT:
-
alternative lengthening of telomeres
- APB:
-
ALT-associated PML body
- DSB:
-
double-strand break
- ECTR:
-
extrachromosomal telomeric repeats
- FISH:
-
fluorescent in situ hybridization
- HPV:
-
human papillioma virus
- HR:
-
homologous recombination
- LFS:
-
Li-Fraumeni syndrome
- PNB:
-
PML nuclear body
- PD:
-
population doubling
- SV40:
-
simion virus 40
- TRD:
-
telomeric rapid deletion
- TERT:
-
telomerase reverse transcriptase
- TR:
-
telomerase RNA
- TRF:
-
terminal restriction fragment.
References
Allshire RC, Dempster M, Hastie ND . 1989 Nucleic Acids Res. 17: 4611–4627
Aogi K, Kitahara K, Buley I, Backdahl M, Tahara H, Sugino T, Tarin D, Goodison S . 1998 Clin. Cancer Res. 4: 1965–1970
Aogi K, Kitahara K, Urquidi V, Tarin D, Goodison S . 1999 Clin. Cancer Res. 5: 2790–2797
Aogi K, Woodman A, Urquidi V, Mangham DC, Tarin D, Goodison S . 2000 Clin. Cancer Res. 6: 4776–4781
Baumann P, Cech TR . 2001 Science 292: 1171–1175
Bertrand P, Rouillard D, Boulet A, Levalois C, Soussi T, Lopez BS . 1997 Oncogene 14: 1117–1122
Biessmann H, Mason JM, Ferry K, d'Hulst M, Valgeirsdottir K, Traverse KL, Pardue M-L . 1990 Cell 61: 663–673
Bischof O, Kim S-H, Irving J, Beresten S, Ellis NA, Campisi J . 2001 J. Cell Biol. 153: 367–380
Bovée JVMG, van den Broek LJCM, Cleton-Jansen A-M, Hogendoorn PCW . 2001 J. Pathol. 193: 354–360
Brosh Jr RM, Karmakar P, Sommers JA, Yang Q, Wang XW, Spillare EA, Harris CC, Bohr VA . 2001 J. Biol. Chem. 276: 35093–35102
Brousset P, Chaouche N, Leprat F, Branet-Brousset F, Trouette H, Zenou RC, Merlio J-P, Delsol G . 1997 J. Clin. Endocrinol. Metab. 82: 4214–4216
Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR . 1997a Nat. Med. 3: 1271–1274
Bryan TM, Englezou A, Dunham MA, Reddel RR . 1998 Exp. Cell Res. 239: 370–378
Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR . 1995 EMBO J. 14: 4240–4248
Bryan TM, Marusic L, Bacchetti S, Namba M, Reddel RR . 1997b Hum. Mol. Genet. 6: 921–926
Bryan TM, Reddel RR . 1997 Eur. J. Cancer 33: 767–773
Buchhop S, Gibson MK, Wang XW, Wagner P, Stürzbecher H-W, Harris CC . 1997 Nucleic Acids Res. 25: 3868–3874
Bucholc M, Park Y, Lustig AJ . 2001 Mol. Cell. Biol. 21: 6559–6573
Cerone MA, Londono-Vallejo JA, Bacchetti S . 2001 Hum. Mol. Genet. 10: 1945–1952
Cheng A-J, Lin J-D, Chang T, Wang T-CV . 1998 Br. J. Cancer 77: 2177–2180
Cohen H, Sinclair DA . 2001 Proc. Natl. Acad. Sci. USA 98: 3174–3179
Cohen S, Regev A, Lavi S . 1997 Oncogene 14: 977–985
Colgin LM, Reddel RR . 1999 Curr. Opin. Genet. Dev. 9: 97–103
de Lange T . 1995 Telomere dynamics and genome instability in human cancer In Telomeres Blackburn EH, Greider CW (eds) Cold Spring Harbor Laboratory Press: New York pp 265–293
de Lange T, Petrini JHJ . 2001 Cold Spring Harb. Symp. Quant. Biol. in press
de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE . 1990 Mol. Cell. Biol. 10: 518–527
Dessain SK, Yu H, Reddel RR, Beijersbergen RL, Weinberg RA . 2000 Cancer Res. 60: 537–541
Dunham MA, Neumann AA, Fasching CL, Reddel RR . 2000 Nat. Genet. 26: 447–450
Ford LP, Zou Y, Pongracz K, Gryaznov SM, Shay JW, Wright WE . 2001 J. Biol. Chem. 276: 32198–32203
Gollahon LS, Kraus E, Wu T-A, Yim SO, Strong LC, Shay JW, Tainsky MA . 1998 Oncogene 17: 709–717
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T . 1999 Cell 97: 503–514
Grobelny JV, Godwin AK, Broccoli D . 2000 J. Cell Sci. 113: 4577–4585
Grobelny JV, Kulp-McEliece M, Broccoli D . 2001 Hum. Mol. Genet. 10: 1953–1961
Guiducci C, Cerone MA, Bacchetti S . 2001 Oncogene 20: 714–725
Hande MP, Balajee AS, Tchirkov A, Wynshaw-Boris A, Lansdorp PM . 2001 Hum. Mol. Genet. 10: 519–528
Hande MP, Samper E, Lansdorp P, Blasco MA . 1999 J. Cell Biol. 144: 589–601
Harley CB . 1997 Ciba Found. Symp. 211: 129–144
Haugen BR, Nawaz S, Markham N, Hashizumi T, Shroyer AL, Werness B, Shroyer KR . 1997 Thyroid 7: 337–342
Henderson S, Allsopp R, Spector D, Wang S-S, Harley C . 1996 J. Cell Biol. 134: 1–12
Herrera E, Martínez C, Blasco MA . 2000 EMBO J. 19: 472–481
Hickson ID, Davies SL, Li J-L, Levitt NC, Mohaghegh P, North PS, Wu L . 2001 Biochem. Soc. Trans. 29: 201–204
Hiraga S, Ohnishi T, Izumoto S, Miyahara E, Kanemura Y, Matsumura H, Arita N . 1998 Cancer Res. 58: 2117–2125
Hoare SF, Bryce LA, Wisman GBA, Burns S, Going JJ, Van der Zee AGJ, Keith WN . 2001 Cancer Res. 61: 27–32
Hodges M, Tissot C, Howe K, Grimwade D, Freemont PS . 1998 Am. J. Hum. Genet. 63: 297–304
Hu P, Beresten SF, van Brabant AJ, Ye T-Z, Pandolfi P-P, Johnson FB, Guarente L, Ellis NA . 2001 Hum. Mol. Genet. 10: 1287–1298
Huang P-H, Pryde FE, Lester D, Maddison RL, Borts RH, Hickson ID, Louis EJ . 2001 Curr. Biol. 11: 125–129
Johnson FB, Marciniak RA, McVey M, Stewart SA, Hahn WC, Guarente L . 2001 EMBO J. 20: 905–913
Kammori M, Takubo K, Nakamura K, Furugouri E, Endo H, Kanauchi H, Mimura Y, Kaminishi M . 2000 Cancer Lett. 159: 175–181
Karow JK, Wu L, Hickson ID . 2000 Curr. Opin. Genet. Dev. 10: 32–38
Katoh M, Katoh M, Kameyama M, Kugoh H, Shimizu M, Oshimura M . 1998 Mol. Carcinog. 21: 17–25
Kilian A, Bowtell DDL, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA . 1997 Hum. Mol. Genet. 6: 2011–2019
Kishi S, Wulf G, Nakamura M, Lu KP . 2001 Oncogene 20: 1497–1508
Kreuzer KN . 2000 Trends Biochem. Sci. 25: 165–173
Lallemand-Breitenbach V, Zhu J, Puvion F, Koken M, Honoré N, Doubeikovsky A, Duprez E, Pandolfi PP, Puvion E, Freemont P, De Thé H . 2001 J. Exp. Med. 193: 1361–1372
Lansdorp PM, Poon S, Chavez E, Dragowska V, Zijlmans M, Bryan T, Reddel R, Egholm M, Bacchetti S, Martens U . 1997 Ciba Found. Symp. 211: 209–218
Le S, Moore JK, Haber JE, Greider CW . 1999 Genetics 152: 143–152
Li B, Lustig AJ . 1996 Genes Dev. 10: 1310–1326
Lipps HJ, Feiler S, Azorin F . 1998 J. Mol. Biol. 283: 1–7
Liu Y, Maizels N . 2000 EMBO rep. 1: 85–90
Lo CY, Lam KY, Chan KT, Luk JM . 1999 Thyroid 9: 1215–1220
Lombard DB, Guarente L . 2000 Cancer Res. 60: 2331–2334
Ludérus ME, van Steensel B, Chong L, Sibon OCM, Cremers FFM, de Lange T . 1996 J. Cell Biol. 135: 867–881
Lundblad V, Blackburn EH . 1993 Cell 73: 347–360
Martens UM, Chavez EA, Poon SS, Schmoor C, Lansdorp PM . 2000 Exp. Cell Res. 256: 291–299
Matthews P, Jones CJ, Skinner J, Haughton M, de Micco C, Wynford-Thomas D . 2001 J. Pathol. 194: 183–193
Maul GG, Negorev D, Bell P, Ishov AM . 2000 J. Struct. Biol. 129: 278–287
McEachern MJ . 2001 Recombinational telomere elongation in the yeast K. lactis In Telomeres and telomerases: cancer and biology Krupp G (ed) Landes Bioscience: Georgetown, TX, USA in press
McEachern MJ, Blackburn EH . 1996 Genes Dev. 10: 1822–1834
Mehle C, Piatyszek MA, Ljungberg B, Shay JW, Roos G . 1996 Oncogene 13: 161–166
Metcalfe JA, Parkhill J, Campbell L, Stacey M, Biggs P, Byrd PJ, Taylor AMR . 1996 Nat. Genet. 13: 350–353
Montalto MC, Phillips JS, Ray FA . 1999 J. Cell. Physiol. 180: 46–52
Muñoz-Jordán JL, Cross GAM, de Lange T, Griffith JD . 2001 EMBO J. 20: 579–588
Murnane JP, Sabatier L, Marder BA, Morgan WF . 1994 EMBO J. 13: 4953–4962
Murti KG, Prescott DM . 1999 Proc. Natl. Acad. Sci. USA 96: 14436–14439
Nakabayashi K, Ogata T, Fujii M, Tahara H, Ide T, Wadhwa R, Kaul SC, Mitsui Y, Ayusawa D . 1997 Exp. Cell Res. 235: 345–353
Niida H, Shinkai Y, Hande MP, Matsumoto T, Takehara S, Tachibana M, Oshimura M, Lansdorp PM, Furuichi Y . 2000 Mol. Cell. Biol. 20: 4115–4127
Noma K, Allis CD, Grewal SIS . 2001 Science 293: 1150–1155
Nosek J, Tomáska L, Fukuhara H, Suyama Y, Kovác L . 1998 Trends Genet. 14: 184–188
Ogata T, Ayusawa D, Namba M, Takahashi E, Oshimura M, Oishi M . 1993 Mol. Cell. Biol. 13: 6036–6043
Ogata T, Oshimura M, Namba M, Fujii M, Oishi M, Ayusawa D . 1995 Jpn. J. Cancer Res. 86: 35–40
Ogino H, Nakabayashi K, Suzuki M, Takahashi E-I, Fujii M, Suzuki T, Ayusawa D . 1998 Biochem. Biophys. Res. Commun. 248: 223–227
Okabe J, Eguchi A, Masago A, Hayakawa T, Nakanishi M . 2000 Hum. Mol. Genet. 9: 2639–2650
Okayasu I, Osakabe T, Fujiwara M, Fukuda H, Kato M, Oshimura M . 1997 Jpn. J. Cancer Res. 88: 965–970
Opitz OG, Suliman Y, Hahn WC, Harada H, Blum HE, Rustgi AK . 2001 J. Clin. Invest. 108: 725–732
Park KH, Rha SY, Kim CH, Kim TS, Yoo NC, Kim JH, Roh JK, Noh SH, Min JS, Lee KS, Kim BS, Chung HC . 1998 Int. J. Oncol. 13: 489–495
Park PU, Defossez P-A, Guarente L . 1999 Mol. Cell. Biol. 19: 3848–3856
Pereira-Smith OM, Smith JR . 1983 Science 221: 964–966
Pereira-Smith OM, Smith JR . 1988 Proc. Natl. Acad. Sci. USA 85: 6042–6046
Perrem K, Bryan TM, Englezou A, Hackl T, Moy EL, Reddel RR . 1999 Oncogene 18: 3383–3390
Perrem K, Colgin LM, Neumann AA, Yeager TR, Reddel RR . 2001 Mol. Cell. Biol. 21: 3862–3875
Reddel RR . 2000 Carcinogenesis 21: 477–484
Reddel RR, Bryan TM, Colgin LM, Perrem KT, Yeager TR . 2001 Radiat. Res. 155: 194–200
Reddel RR, Bryan TM, Murnane JP . 1997 Biochemistry (Mosc) 62: 1254–1262
Regev A, Cohen S, Cohen E, Bar-Am I, Lavi S . 1998 Oncogene 17: 3455–3461
Rizki A, Lundblad V . 2001 Nature 411: 713–716
Rogan EM, Bryan TM, Hukku B, Maclean K, Chang ACM, Moy EL, Englezou A, Warneford SG, Dalla-Pozza L, Reddel RR . 1995 Mol. Cell. Biol. 15: 4745–4753
Roth CW, Kobeski F, Walter MF, Biessmann H . 1997 Mol. Cell. Biol. 17: 5176–5183
Ruggero D, Wang Z-G, Pandolfi PP . 2000 BioEssays 22: 827–835
Saji M, Westra WH, Chen H, Umbricht CB, Tuttle RM, Box MF, Udelsman R, Sukumar S, Zeiger MA . 1997 Surgery 122: 1137–1140
Saji M, Xydas S, Westra WH, Liang C-K, Clark DP, Udelsman R, Umbricht CB, Sukumar S, Zeiger MA . 1999 Clin. Cancer Res. 5: 1483–1489
Scheel C, Schaefer K-L, Jauch A, Keller M, Wai D, Brinkschmidt C, van Valen F, Boecker W, Dockhorn-Dworniczak B, Poremba C . 2001 Oncogene 20: 3835–3844
Schneider-Stock R, Epplen JT, Walter H, Radig K, Rys J, Epplen C, Hoang-Vu C, Niezabitowski A, Roessner A . 1999 Mol. Carcinog. 24: 144–151
Shay JW, Bacchetti S . 1997 Eur. J. Cancer 33: 787–791
Shay JW, Tomlinson G, Piatyszek MA, Gollahon LS . 1995 Mol. Cell. Biol. 15: 425–432
Shen Z, Pardington-Purtymun PE, Comeaux JC, Moyzis RK, Chen DJ . 1996 Genomics 36: 271–279
Shore D . 2001 Curr. Opin. Genet. Dev. 11: 189–198
Sinclair DA, Guarente L . 1997 Cell 91: 1033–1042
Small MB, Hubbard K, Pardinas JR, Marcus AM, Dhanaraj SN, Sethi KA . 1996 J. Cell. Physiol. 168: 727–736
Smith J, Zou H, Rothstein R . 2000 Biochimie 82: 71–78
Sprung CN, Bryan TM, Reddel RR, Murnane JP . 1997 Mutat. Res. 379: 177–184
Strahl C, Blackburn EH . 1996 Mol. Cell. Biol. 16: 53–65
Sugawara N, Ivanov EL, Fishman-Lobell J, Ray BL, Wu X, Haber JE . 1995 Nature 373: 84–86
Sugihara S, Mihara K, Marunouchi T, Inoue H, Namba M . 1996 Hum. Genet. 97: 1–6
Sugimoto M, Ide T, Goto M, Furuichi Y . 1999 Mech. Ageing Dev. 107: 51–60
Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S . 1998 EMBO J. 17: 5497–5508
Tanaka K, Nishide J, Okazaki K, Kato H, Niwa O, Nakagawa T, Matsuda H, Kawamukai M, Murakami Y . 1999 Mol. Cell. Biol. 19: 8660–8672
Teng S-C, Chang J, McCowan B, Zakian VA . 2000 Mol. Cell 6: 947–952
Teng S-C, Zakian VA . 1999 Mol. Cell. Biol. 19: 8083–8093
Thompson LH, Schild D . 2001 Mutat. Res. 477: 131–153
Tokutake Y, Matsumoto T, Watanabe T, Maeda S, Tahara H, Sakamoto S, Niida H, Sugimoto M, Ide T, Furuichi Y . 1998 Biochem. Biophys. Res. Commun. 247: 765–772
Tomaska L, Nosek J, Makhov AM, Pastorakova A, Griffith JD . 2000 Nucleic Acids Res. 28: 4479–4487
Tommerup H, Dousmanis A, de Lange T . 1994 Mol. Cell. Biol. 14: 5777–5785
Tong W-M, Hande MP, Lansdorp PM, Wang Z-Q . 2001 Mol. Cell. Biol. 21: 4046–4054
Tsutsui T, Fujino T, Kodama S, Tainsky MA, Boyd J, Barrett JC . 1995 Carcinogenesis 16: 25–34
Tsutsui T, Tanaka Y, Matsudo Y, Hasegawa K, Fujino T, Kodama S, Barrett JC . 1997 Mol. Carcinog. 18: 7–18
Vogt M, Haggblom C, Yeargin J, Christiansen-Weber T, Haas M . 1998 Cell Growth Differ. 9: 139–146
Wahl GM . 1989 Cancer Res. 49: 1333–1340
Wang XW, Tseng A, Ellis NA, Spillare EA, Linke SP, Robles AI, Seker H, Yang Q, Hu P, Beresten S, Bemmels NA, Garfield S, Harris CC . 2001 J. Biol. Chem. 276: 32948–32955
Wen J, Cong Y-S, Bacchetti S . 1998 Hum. Mol. Genet. 7: 1137–1141
Whitaker NJ, Bryan TM, Bonnefin P, Chang ACM, Musgrove EA, Braithwaite AW, Reddel RR . 1995 Oncogene 11: 971–976
Wold MS . 1997 Annu. Rev. Biochem. 66: 61–92
Wotton D, Shore D . 1997 Genes Dev. 11: 748–760
Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW . 1997 Genes Dev. 11: 2801–2809
Wu G, Lee W-H, Chen P-L . 2000 J. Biol. Chem. 275: 30618–30622
Wu L, Davies SL, Levitt NC, Hickson ID . 2001 J. Biol. Chem. 276: 19375–19381
Xia SJ, Shammas MA, Shmookler Reis RJ . 1996 Mutat. Res. 364: 1–11
Yan P, Coindre J-M, Benhattar J, Bosman FT, Guillou L . 1999 Cancer Res. 59: 3166–3170
Yankiwski V, Marciniak RA, Guarente L, Neff NF . 2000 Proc. Natl. Acad. Sci. USA 97: 5214–5219
Yashima K, Vuitch F, Gazdar AF, Fahey III TJ . 1997 Surgery 122: 1141–1145
Yeager TR, Neumann AA, Englezou A, Huschtscha LI, Noble JR, Reddel RR . 1999 Cancer Res. 59: 4175–4179
Yeh ETH, Gong L, Kamitani T . 2000 Gene 248: 1–14
Yoo J, Robinson RA . 2000 Arch. Pathol. Lab. Med. 124: 393–397
Zhong S, Salomoni P, Pandolfi PP . 2000 Nat. Cell Biol. 2: E85–E90
Zhu X-D, Küster B, Mann M, Petrini JHJ, de Lange T . 2000 Nat. Genet. 25: 347–352
Acknowledgements
The authors thank Clare Fasching for comments on the manuscript. Work in the authors' laboratory is supported by Cancer Council NSW and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henson, J., Neumann, A., Yeager, T. et al. Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 (2002). https://doi.org/10.1038/sj.onc.1205058
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205058
Keywords
This article is cited by
-
The crosstalk between telomeres and DNA repair mechanisms: an overview to mammalian somatic cells, germ cells, and preimplantation embryos
Journal of Assisted Reproduction and Genetics (2024)
-
A pan-sarcoma landscape of telomeric content shows that alterations in RAD51B and GID4 are associated with higher telomeric content
npj Genomic Medicine (2023)
-
Alternative lengthening of telomeres (ALT) cells viability is dependent on C-rich telomeric RNAs
Nature Communications (2023)
-
Hormonal regulation of telomerase activity and hTERT expression in steroid-regulated tissues and cancer
Cancer Cell International (2022)
-
A pan-cancer landscape of telomeric content shows that RAD21 and HGF alterations are associated with longer telomeres
Genome Medicine (2022)