Abstract
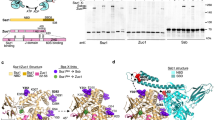
Ribosome-associated J protein–Hsp70 chaperones promote nascent-polypeptide folding and normal translational fidelity. The J protein Zuo1 is known to span the ribosomal subunits, but understanding of its function is limited. Here we present new structural and cross-linking data allowing more precise positioning of Saccharomyces cerevisiae Zuo1 near the 60S polypeptide-exit site and suggesting interactions of Zuo1 with the ribosomal protein eL31 and 25S rRNA helix 24. The junction between the 60S-interacting and subunit-spanning helices is a hinge that positions Zuo1 on the 40S yet accommodates subunit rotation. Interaction between the Zuo1 C terminus and 40S occurs via 18S rRNA expansion segment 12 (ES12) of helix 44, which originates at the decoding site. Deletions in either ES12 or the Zuo1 C terminus alter readthrough of stop codons and –1 frameshifting. Our study offers insight into how this cotranslational chaperone system may monitor decoding-site activity and nascent-polypeptide transit, thereby coordinating protein translation and folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim, Y.E., Hipp, M.S., Bracher, A., Hayer-Hartl, M. & Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 (2013).
Gloge, F., Becker, A.H., Kramer, G. & Bukau, B. Co-translational mechanisms of protein maturation. Curr. Opin. Struct. Biol. 24, 24–33 (2014).
Wrobel, L. et al. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524, 485–488 (2015).
Wang, X. & Chen, X.J. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 524, 481–484 (2015).
Korennykh, A. & Walter, P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 28, 251–277 (2012).
Sherman, M.Y. & Qian, S.B. Less is more: improving proteostasis by translation slow down. Trends Biochem. Sci. 38, 585–591 (2013).
Nelson, R.J., Ziegelhoffer, T., Nicolet, C., Werner-Washburne, M. & Craig, E.A. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71, 97–105 (1992).
Preissler, S. & Deuerling, E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274–283 (2012).
Yan, W. et al. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 17, 4809–4817 (1998).
Hundley, H.A., Walter, W., Bairstow, S. & Craig, E.A. Human Mpp11 J protein: ribosome-tethered molecular chaperones are ubiquitous. Science 308, 1032–1034 (2005).
Kampinga, H.H. & Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 (2010).
Huang, P., Gautschi, M., Walter, W., Rospert, S. & Craig, E.A. The Hsp70 Ssz1 modulates the function of the ribosome-associated J-protein Zuo1. Nat. Struct. Mol. Biol. 12, 497–504 (2005).
Clerico, E.M., Tilitsky, J.M., Meng, W. & Gierasch, L.M. How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J. Mol. Biol. 427, 1575–1588 (2015).
Willmund, F. et al. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152, 196–209 (2013).
Gautschi, M. et al. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin. Proc. Natl. Acad. Sci. USA 98, 3762–3767 (2001).
Gautschi, M., Mun, A., Ross, S. & Rospert, S. A functional chaperone triad on the yeast ribosome. Proc. Natl. Acad. Sci. USA 99, 4209–4214 (2002).
Otto, H. et al. The chaperones MPP11 and Hsp70L1 form the mammalian ribosome-associated complex. Proc. Natl. Acad. Sci. USA 102, 10064–10069 (2005).
Fiaux, J. et al. Structural analysis of the ribosome-associated complex (RAC) reveals an unusual Hsp70/Hsp40 interaction. J. Biol. Chem. 285, 3227–3234 (2010).
Conz, C. et al. Functional characterization of the atypical Hsp70 subunit of yeast ribosome-associated complex. J. Biol. Chem. 282, 33977–33984 (2007).
Jaiswal, H. et al. The chaperone network connected to human ribosome-associated complex. Mol. Cell. Biol. 31, 1160–1173 (2011).
Rakwalska, M. & Rospert, S. The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae. Mol. Cell. Biol. 24, 9186–9197 (2004).
Caliskan, N., Peske, F. & Rodnina, M.V. Changed in translation: mRNA recoding by -1 programmed ribosomal frameshifting. Trends Biochem. Sci. 40, 265–274 (2015).
Muldoon-Jacobs, K.L. & Dinman, J.D. Specific effects of ribosome-tethered molecular chaperones on programmed -1 ribosomal frameshifting. Eukaryot. Cell 5, 762–770 (2006).
Peisker, K. et al. Ribosome-associated complex binds to ribosomes in close proximity of Rpl31 at the exit of the polypeptide tunnel in yeast. Mol. Biol. Cell 19, 5279–5288 (2008).
Leidig, C. et al. Structural characterization of a eukaryotic chaperone—the ribosome-associated complex. Nat. Struct. Mol. Biol. 20, 23–28 (2013).
Zhang, Y. et al. Structural basis for interaction of a cotranslational chaperone with the eukaryotic ribosome. Nat. Struct. Mol. Biol. 21, 1042–1046 (2014).
Ducett, J.K. et al. Unfolding of the C-terminal domain of the J-protein Zuo1 releases autoinhibition and activates Pdr1-dependent transcription. J. Mol. Biol. 425, 19–31 (2013).
Kaschner, L.A., Sharma, R., Shrestha, O.K., Meyer, A.E. & Craig, E.A. A conserved domain important for association of eukaryotic J-protein co-chaperones Jjj1 and Zuo1 with the ribosome. Biochim. Biophys. Acta 1853, 1035–1045 (2015).
Albanèse, V., Reissmann, S. & Frydman, J. A ribosome-anchored chaperone network that facilitates eukaryotic ribosome biogenesis. J. Cell Biol. 189, 69–81 (2010).
Wai, H.H., Vu, L., Oakes, M. & Nomura, M. Complete deletion of yeast chromosomal rDNA repeats and integration of a new rDNA repeat: use of rDNA deletion strains for functional analysis of rDNA promoter elements in vivo. Nucleic Acids Res. 28, 3524–3534 (2000).
Greber, B.J., Boehringer, D., Montellese, C. & Ban, N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat. Struct. Mol. Biol. 19, 1228–1233 (2012).
Hundley, H. et al. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc. Natl. Acad. Sci. USA 99, 4203–4208 (2002).
Svidritskiy, E., Brilot, A.F., Koh, C.S., Grigorieff, N. & Korostelev, A.A. Structures of yeast 80S ribosome-tRNA complexes in the rotated and nonrotated conformations. Structure 22, 1210–1218 (2014).
Pechmann, S., Willmund, F. & Frydman, J. The ribosome as a hub for protein quality control. Mol. Cell 49, 411–421 (2013).
Hilal, T. & Spahn, C.M. Ribosome rescue and protein quality control in concert. Mol. Cell 57, 389–390 (2015).
Shen, P.S. et al. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347, 75–78 (2015).
Youngman, E.M., Cochella, L., Brunelle, J.L., He, S. & Green, R. Two distinct conformations of the conserved RNA-rich decoding center of the small ribosomal subunit are recognized by tRNAs and release factors. Cold Spring Harb. Symp. Quant. Biol. 71, 545–549 (2006).
Carter, A.P. et al. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348 (2000).
Zaher, H.S. & Green, R. Fidelity at the molecular level: lessons from protein synthesis. Cell 136, 746–762 (2009).
Nakatogawa, H. & Ito, K. The ribosomal exit tunnel functions as a discriminating gate. Cell 108, 629–636 (2002).
Lin, P.J., Jongsma, C.G., Pool, M.R. & Johnson, A.E. Polytopic membrane protein folding at L17 in the ribosome tunnel initiates cyclical changes at the translocon. J. Cell Biol. 195, 55–70 (2011).
Fulle, S. & Gohlke, H. Statics of the ribosomal exit tunnel: implications for cotranslational peptide folding, elongation regulation, and antibiotics binding. J. Mol. Biol. 387, 502–517 (2009).
Zhang, Y., Wölfle, T. & Rospert, S. Interaction of nascent chains with the ribosomal tunnel proteins Rpl4, Rpl17, and Rpl39 of Saccharomyces cerevisiae. J. Biol. Chem. 288, 33697–33707 (2013).
Wilson, D.N. & Beckmann, R. The ribosomal tunnel as a functional environment for nascent polypeptide folding and translational stalling. Curr. Opin. Struct. Biol. 21, 274–282 (2011).
Pool, M.R. A trans-membrane segment inside the ribosome exit tunnel triggers RAMP4 recruitment to the Sec61p translocase. J. Cell Biol. 185, 889–902 (2009).
Fluitt, A., Pienaar, E. & Viljoen, H. Ribosome kinetics and aa-tRNA competition determine rate and fidelity of peptide synthesis. Comput. Biol. Chem. 31, 335–346 (2007).
Wild, K., Halic, M., Sinning, I. & Beckmann, R. SRP meets the ribosome. Nat. Struct. Mol. Biol. 11, 1049–1053 (2004).
Elvekrog, M.M. & Walter, P. Dynamics of co-translational protein targeting. Curr. Opin. Chem. Biol. 29, 79–86 (2015).
Prunuske, A.J., Waltner, J.K., Kuhn, P., Gu, B. & Craig, E.A. Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1. Proc. Natl. Acad. Sci. USA 109, 472–477 (2012).
Richly, H. et al. Transcriptional activation of polycomb-repressed genes by ZRF1. Nature 468, 1124–1128 (2010).
Gracheva, E. et al. ZRF1 mediates remodeling of E3 ligases at DNA lesion sites during nucleotide excision repair. J. Cell Biol. 213, 185–200 (2016).
Blommel, P.G., Becker, K.J., Duvnjak, P. & Fox, B.G. Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition. Biotechnol. Prog. 23, 585–598 (2007).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 47, 5.6 (2014).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
James, P., Pfund, C. & Craig, E.A. Functional specificity among Hsp70 molecular chaperones. Science 275, 387–389 (1997).
Sikorski, R.S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 (1989).
Mumberg, D., Müller, R. & Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122 (1995).
Mountain, H.A., Byström, A.S., Larsen, J.T. & Korch, C. Four major transcriptional responses in the methionine/threonine biosynthetic pathway of Saccharomyces cerevisiae. Yeast 7, 781–803 (1991).
Nemoto, N. et al. Yeast 18 S rRNA is directly involved in the ribosomal response to stringent AUG selection during translation initiation. J. Biol. Chem. 285, 32200–32212 (2010).
Krishnamurthy, M. et al. Caught in the act: covalent cross-linking captures activator-coactivator interactions in vivo. ACS Chem. Biol. 6, 1321–1326 (2011).
Ting, S.Y., Schilke, B.A., Hayashi, M. & Craig, E.A. Architecture of the TIM23 inner mitochondrial translocon and interactions with the matrix import motor. J. Biol. Chem. 289, 28689–28696 (2014).
Eisenman, H.C. & Craig, E.A. Activation of pleiotropic drug resistance by the J-protein and Hsp70-related proteins, Zuo1 and Ssz1. Mol. Microbiol. 53, 335–344 (2004).
Stone, D.E. & Craig, E.A. Self-regulation of 70-kilodalton heat shock proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 10, 1622–1632 (1990).
Halladay, J.T. & Craig, E.A. A heat shock transcription factor with reduced activity suppresses a yeast HSP70 mutant. Mol. Cell. Biol. 15, 4890–4897 (1995).
Vilardell, J. & Warner, J.R. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell. Biol. 17, 1959–1965 (1997).
Salas-Marco, J. & Bedwell, D.M. Discrimination between defects in elongation fidelity and termination efficiency provides mechanistic insights into translational readthrough. J. Mol. Biol. 348, 801–815 (2005).
Kramer, E.B., Vallabhaneni, H., Mayer, L.M. & Farabaugh, P.J. A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA 16, 1797–1808 (2010).
Namy, O., Hatin, I. & Rousset, J.P. Impact of the six nucleotides downstream of the stop codon on translation termination. EMBO Rep. 2, 787–793 (2001).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Jones, D.T., Taylor, W.R. & Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282 (1992).
Acknowledgements
We thank A. Senes, J. Keck and S. Butcher for helpful discussions during the course of this work; D. Bedwell (University of Alabama), P. Farabaugh (University of Maryland, Baltimore County) and J. Dinman (University of Maryland) for dual-luciferase plasmids; K. Asano (Kansas State University) for the rDNA-deletion strain; and J. Warner (Albert Einstein College of Medicine) for anti-uL3. This work was supported by National Institutes of Health grants GM031107 and GM027870 (E.A.C.). C.A.B. was supported by NIH grants GM094584, GM094622 and GM098248. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. GM/CA@APS has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006).
Author information
Authors and Affiliations
Contributions
K.L. performed in vivo cross-linking experiments, analyses of ribosome association, generation of mutants and other molecular-biological experiments. R.S. performed in vivo misreading experiments, analyses of ribosome association, generation of mutants and other molecular-biological experiments. O.K.S. performed crystallographic analysis, analyses of ribosome association and generation of mutants. C.A.B. performed crystallographic analysis. E.A.C. oversaw all aspects of the experiments and manuscript preparation. All authors participated in interpreting the data and writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
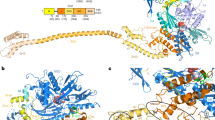
Supplementary Figure 1 Structure and sequence analyses of the Zuotin homology domain (ZHD).
(a) Superimposition of the two molecules of Zuo1166-303 in the asymmetric unit. Chain A and B are colored blue and purple, respectively. No electron density was observed for residues 166-168 of chain A and 166-167 of chain B. The RMSD of alpha carbon positions is 2.8 Å. The major difference between chain A and B is the loop between helix II and helix III (residues 228-245). When these residues are excluded, the RMSD is 1.4 Å. Chain A and chain B have an average B-value of 35 and 40, respectively. Chain A was used throughout. Figure generated by PyMol. (b) Representative stereo image of the 2FO-FC electron density map of ZHD calculated after the final refinement contoured at 1.0 σ. Residues Tyr221, Asp283, Pro284 and Arg285 forming the hinge at the junction between ZHD and middle domain shown in stick representation. Figure generated by PyMol. (c) Model structures of the ZHD. S. cerevisiae and human Zuo1 and Jjj1 sequences were aligned by Clustal Omega. The resulting alignment was used for generating model structures by Modeller, employing the S. cerevisiae Zuo1 ZHD structure as a template. The ZHD of yeast Jjj1 contains an insertion between helix I and helix II that is not conserved in higher eukaryotes such as humans. This insertion and the lower conservation of helix I led to an initial definition of the ZHD (i.e. 205-285 of Zuo1) that lacked helix I. (d) Phylogenetic analysis of Zuo1 and Jjj1 and conservation of residues forming the hinge region in the two proteins. Amino acid sequences of Zuo1 and Jjj1 orthologs from the indicated organisms were aligned using ClustalW. The trees were constructed using the Maximum Likelihood method based on the JTT matrix-based model conducted using MEGA7. The three residues present at the hinge of Zuo1 and Jjj1 [Asp283(D), Pro284(P), Arg285(R) and Asp257(D), Lys258(K), Arg259(R), respectively] are shown indicating a high conservation of salt-bridge partners aspartic acid and arginine flanking a relatively variable residue.
Supplementary Figure 2 Interaction of Zuo1 ZHD with the 60S subunit.
(a) Bpa was incorporated into eL31a-HA and eL22a-HA at positions highlighted. Sites that cross-linked shown in ball and stick representation; those that did not by stick only. (b) Site-specific cross-linking between eL31a-HA and Zuo1. Cells expressing indicated variants were grown in the presence of Bpa and exposed to UV light (+), or as a control not exposed (-), before lysis. Samples, run in parallel, were separated by gel electrophoresis and analyzed using antibodies specific for Zuo1 or HA tag (eL31a). Migration of molecular weight standards (left, in kDa) and Zuo1 and HA reactive bands (right, arrows) are indicated. Asterisk indicates crosslink between Zuo1 and Ssz1 (see panel d for explanation). Crosslinking was carried out with two independent yeast transformants, with similar results. (c) Residues of Zuo1 ZHD tested by Bpa cross-linking are highlighted (top, cyan). Sites that crosslinked are shown in ball and stick representation; those that did not, stick representation only. Cross-linking to eL31a was analyzed as in b except Zuo1-Asp262Bpa samples were electrophoresed in gels having 15%, rather than 10%, acrylamide. Crosslinking was carried out with two independent yeast transformants, with similar results. (d) Control of site-specific Bpa cross-linking experiments for unidentified band indicated by asterisks in panel b above, and in Fig. 2b. Cells expressing HA-tagged eL31a wild type (WT) and Arg79Bpa (top) and ∆ssz1 cells carrying pRS315-Ssz1 or a pRS315-Ssz1 variant with stop codon altered to TGA from TAG (bottom) were grown in the presence of Bpa. Cross-linking was analyzed as in b except antibodies specific for Zuo1 or Ssz1 were used for immunoblot analysis. Band reacting with both Zuo1 and Ssz1 antibody indicated with asterisk (*). Zuo1 and eL31a cross-linked band indicated with arrow. Migration of molecular weight standards is indicated in kDa (left). (e) Validation of antibody directed against Zuo1 residues 166-284. Yeast cell lysates of wild type cells of DS10 (WT strain) or ∆zuo1 cells expressing Zuo1 (WT), no Zuo1 (---) or truncation Zuo1 variant lacking the N-terminal 91 residues (92-433) were subjected to electrophoresis and immunoblot analysis. Antibody raised to Zuo1 residues from 166 to 368 tagged with GST was used. (f) Lysates of cells expressing wild type (WT) or variant Zuo1 with alterations Asp262/Thr266/Val273Ala (DTV) were centrifuged through sucrose cushions. Supernatant (S) and pellet (P) fractions, as well as total lysates (T), were analyzed by immunoblotting with indicated antibodies after SDS-PAGE. One representative immunoblot (from three independent experiments, performed with different yeast cultures) is shown. (b-e) Uncropped gel images are shown in Supplementary Data Set 1. (g) Secondary structure of 25S rRNA. Close-up view of H24 and H59 shown, with red dashed lines indicating deletions. Adapted from http://apollo.chemistry.gatech.edu/RibosomeGallery.
Supplementary Figure 3 Docking of Zuo1 ZHD to the 60S subunit.
The atomic structure of the ribosome (PDB 3J78) was fit to the cryo-EM map (gray surface) of Zuo1-Ssz1 bound ribosome. Zuo1 ZHD 169-303 was then manually docked to eL31, positioning the cross-linked residues (Thr266 and Val273 of Zuo1 and Val7, Arg79 and Glu81 of eL31, shown as spheres) in close proximity. After manually pointing the Arg247/Arg251 (spheres) at the tip of helix III, either towards H24 (H24 model, ZHD purple) or H59 and eL22 (eL22/H59 model, ZHD red), Zuo1 ZHD was fit to the cryo-EM map by rigid-body docking. PTE: Polypeptide tunnel exit.
Supplementary Figure 4 Interaction of the C terminus of Zuo1 with the 40S subunit.
(a) Secondary structure and location of expansion segment 12 (ES12) of rRNA H44. Secondary structure of 18S rRNA was adapted from http://apollo.chemistry.gatech.edu/RibosomeGallery. Deleted base pairs of ES12 are indicated with red dashed lines in the close-up view. (b) Stability of interaction of Zuo1 and MD variant with ribosomes. ∆zuo1 cells expressing either wild type Zuo1 (WT) or variant Zuo1Lys341/342/344Ala (K341/342/344A3) were lysed and centrifuged through sucrose cushions. Equivalent amounts of total lysate (T), supernatant (S) and pellet (P) fractions were subjected to electrophoresis and immunoblot analysis. Antibodies specific for Zuo1, and for uL3 and Ssa, as controls for ribosomes and a soluble protein, respectively were used. One representative immunoblot from three independent experiments, performed with different yeast transformants, is shown. Uncropped gel images are shown in Supplementary Data Set 1. (c) Expression of Zuo1 from the MET3 promoter. ∆zuo1 cells harboring a plasmid with no insert (---), Zuo1 expressed from its endogenous promoter (PZUO1) or from the repressible MET3 promoter (PMET3) were grown in selective minimal glucose medium supplemented with 400 μg/ml methionine to 0.5 OD units. Equal number of cells were harvested and indicated amounts (1x, 2x, 10x or 20x) of whole cell lysates were subjected to electrophoresis and immunoblotting with antibody specific for Zuo1 or a cytoplasmic protein, Ssa, as a control. Zuo1 levels from PZUO1 and PMET3 were analyzed at least three times using different yeast transformants; one representative immunoblot is shown. Uncropped gel images are shown in Supplementary Data Set 1. (d) Growth analysis of strains with a deletion of ZUO1 (∆zuo1) or a deletion of all chromosomal rDNA genes, and expressing only one rDNA gene from a plasmid (rRNAsc). Ten-fold serial dilutions of yeast cells were spotted on selective medium plates and incubated at 30°C for 2 days or at 23°C for 3.5 days. Plates supplemented with 0.75 M NaCl (+cation) were incubated at 30°C for 3.5 days. ∆zuo1 cells contained plasmids encoding either wild-type Zuo1 (WT), no insert (---), Zuo1Lys348/352/353Ala (K3A3), residues 1-310 of Zuo1 (1-310) or reduced levels of Zuo1 expressed from the MET3 promoter (Low). In the case of rRNAsc, cells harboring either wild type (WT) rDNA or a copy of rDNA with a deletion of 10 base pairs from the stem of ES12 (ES12∆10) are shown. One representative experiment (from three independent experiments, performed with different yeast cultures) is shown. (e) in vivo analyses of readthrough and -1 frameshifting of wild type Zuo1 and Zuo1His128Gln, altering the conserved HPD motif of the J-domain. Relative levels in cells expressing wild type Zuo1 set at 1. Data shown are means and s.e.m. from values obtained for three independent yeast transformants each assayed in triplicate. (f) Effects of alteration of amino acids forming the ZHD-MD hinge on cell growth. ∆zuo1 cells that contained plasmids encoding either wild type Zuo1 (WT), no insert (---), or indicated variants were used. Cell growth was analyzed as in d.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 1241 kb)
Supplementary Data Set 1
Uncropped western blots (PDF 804 kb)
Rights and permissions
About this article
Cite this article
Lee, K., Sharma, R., Shrestha, O. et al. Dual interaction of the Hsp70 J-protein cochaperone Zuotin with the 40S and 60S ribosomal subunits. Nat Struct Mol Biol 23, 1003–1010 (2016). https://doi.org/10.1038/nsmb.3299
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3299
This article is cited by
-
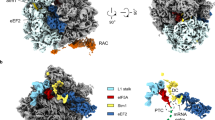
Pathway of Hsp70 interactions at the ribosome
Nature Communications (2021)
-
The ribosome-associated complex RAC serves in a relay that directs nascent chains to Ssb
Nature Communications (2020)
-
The Hsp70 chaperone network
Nature Reviews Molecular Cell Biology (2019)
-
Structural insights into a unique Hsp70-Hsp40 interaction in the eukaryotic ribosome-associated complex
Nature Structural & Molecular Biology (2017)
-
The Hsp70 homolog Ssb affects ribosome biogenesis via the TORC1-Sch9 signaling pathway
Nature Communications (2017)