Abstract
Individual microRNAs (miRNAs) can target hundreds of mRNAs forming networks of presumably cooperating genes. To test this presumption, we functionally screened miRNAs and their targets in the context of dedifferentiation of mouse fibroblasts to induced pluripotent stem cells (iPSCs). Along with the miR-302–miR-294 family, the miR-181 family arose as a previously unidentified enhancer of the initiation phase of reprogramming. Endogenous miR-181 miRNAs were transiently elevated with the introduction of Pou5f1 (also known as Oct4), Sox2 and Klf4 (referred to as OSK), and miR-181 inhibition diminished iPSC colony formation. We tested the functional contribution of 114 individual targets of the two families, revealing 25 genes that normally suppress initiation. Coinhibition of targets cooperatively promoted both the frequency and kinetics of OSK-induced reprogramming. These data establish two of the largest functionally defined networks of miRNA-mRNA interactions and reveal previously unidentified relationships among genes that act together to suppress early stages of reprogramming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Fabian, M.R. & Sonenberg, N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat. Struct. Mol. Biol. 19, 586–593 (2012).
Bartel, D.P. Review MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Baek, D. et al. The impact of microRNAs on protein output. Nature 455, 64–71 (2008).
Selbach, M. et al. Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 (2008).
Guo, H., Ingolia, N.T., Weissman, J.S. & Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Lim, L.P. et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 (2005).
Leung, A.K. et al. Genome-wide identification of Ago2 binding sites from mouse embryonic stem cells with and without mature microRNAs. Nat. Struct. Mol. Biol. 18, 237–244 (2011).
Subramanyam, D. & Blelloch, R. From microRNAs to targets: pathway discovery in cell fate transitions. Curr. Opin. Genet. Dev. 21, 498–503 (2011).
Christodoulou, F. et al. Ancient animal microRNAs and the evolution of tissue identity. Nature 463, 1084–1088 (2010).
Farh, K.K. et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310, 1817–1821 (2005).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Samavarchi-Tehrani, P. et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 (2010).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
Polo, J.M. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 (2012).
Judson, R.L., Babiarz, J.E., Venere, M. & Blelloch, R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat. Biotechnol. 27, 459–461 (2009).
Subramanyam, D. et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 29, 443–448 (2011).
Liao, B. et al. MicroRNA cluster 302–367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J. Biol. Chem. 286, 17359–17364 (2011).
Anokye-Danso, F. et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8, 376–388 (2011).
Miyoshi, N. et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 8, 633–638 (2011).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Hu, S. et al. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells 31, 259–268 (2013).
Li, Z., Yang, C., Nakashima, K. & Rana, T.M. Small RNA-mediated regulation of iPS cell generation. EMBO J. 30, 823–834 (2011).
Lin, S.-L. et al. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 39, 1054–1065 (2011).
Wernig, M., Meissner, A., Cassady, J.P. & Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2, 10–12 (2008).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
Blelloch, R., Venere, M., Yen, J. & Ramalho-Santos, M. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell 1, 245–247 (2007).
Zhang, X.D. et al. The use of strictly standardized mean difference for hit selection in primary RNA interference high-throughput screening experiments. J. Biomol. Screen. 12, 497–509 (2007).
Pfaff, N. et al. miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO Rep. 12, 1153–1159 (2011).
Houbaviy, H.B., Murray, M.F. & Sharp, P.A. Embryonic stem cell-specific MicroRNAs. Dev. Cell 5, 351–358 (2003).
Marson, A. et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134, 521–533 (2008).
Chen, J. et al. Synergetic cooperation of microRNAs with transcription factors in iPS cell generation. PLoS ONE 7, e40849 (2012).
Melton, C., Judson, R.L. & Blelloch, R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–626 (2010).
O'loghlen, A. et al. MicroRNA Regulation of Cbx7 Mediates a Switch of Polycomb Orthologs during ESC Differentiation. Cell. Stem Cell 10, 33–46 (2012).
Shi, R. & Chiang, V. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques 39, 519–525 (2005).
Dunning, M.J., Smith, M.L., Ritchie, M.E. & Tavaré, S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics 23, 2183–2184 (2007).
Smyth, G.K. Limma: linear models for microarray data. Bioinformatics and Computational Biology Solutions using R and Bioconductor 397–420 (2005).
Poliseno, L. et al. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci. Signal. 3, ra29 (2010).
Cichocki, F. et al. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J. Immunol. 187, 6171–6175 (2011).
Ji, J. et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50, 472–480 (2009).
Wang, Y. et al. Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 30, 1470–1478 (2011).
Wang, B. et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 29, 1787–1797 (2010).
Grimson, A. et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 (2007).
Mikkelsen, T. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Wang, Y. et al. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 40, 1478–1483 (2008).
Marson, A. et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3, 132–135 (2008).
Maherali, N. & Hochedlinger, K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 19, 1718–1723 (2009).
Ichida, J.K. et al. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog. Stem Cells 5, 491–503 (2009).
Redmer, T. et al. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 12, 720–726 (2011).
Veeman, M.T., Slusarski, D.C., Kaykas, A., Louie, S.H. & Moon, R.T. Zebrafish Prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 (2003).
Kohn, A.D. et al. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273, 11937–11943 (1998).
Yu, Y. et al. Stimulation of somatic cell reprogramming by Eras-Akt-Foxo1 signaling axis. Stem Cells doi:10.1002/stem.1447 (14 June 2013).
Liao, J. et al. Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells. Mol. Ther. 21, 1242–1250 (2013).
Menendez, S., Camus, S. & Belmonte, J.C.I. p53: Guardian of reprogramming. Cell Cycle 9, 3887–3891 (2010).
Vazquez-Martin, A. et al. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 11, 974–989 (2012).
Jin, L. et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature 482, 495–500 (2012).
Jayawardena, T.M. et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473 (2012).
Yoo, A.S. et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 (2011).
Nam, Y. et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. USA 110, 5588–5593 (2013).
Park, C.Y., Choi, Y.S. & McManus, M.T. Analysis of microRNA knockouts in mice. Hum. Mol. Genet. 19, R169–R175 (2010).
Ebert, M.S. & Sharp, P.A. Roles for MicroRNAs in conferring robustness to biological processes. Cell 149, 515–524 (2012).
Acknowledgements
We thank C. Belair, M. Cook, R. Krishnakumar, M. La Russa, M. Shveygert and other members of the Blelloch laboratory for critical reading of the manuscript, A. Amiet (Dharmacon Thermo Scientific) for providing miRNA and siRNA libraries, M. McMahon (University of California, San Francisco) for AKT expression constructs, H. Zhang for assistance with the high-content analysis, J. Paquette, R. Bell and A. Diaz for advice concerning our statistical methods, A. Shenoy for assistance with bioinformatics, and M. Kissner for assistance with flow cytometry. This work was supported by funds to R.B. from US National Institutes of Health (R01:GM101180), the Leona M. and Harry B. Helmsley Charitable Trust (09PG-T1D002) and the California Institute of Regenerative Medicine (RN2-00906-1). R.L.J. was supported by a US National Science Foundation graduate research fellowship.
Author information
Authors and Affiliations
Contributions
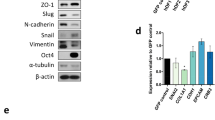
R.L.J. contributed to data in Figures 1,2,3,4a–e,5,6,7a,f,g and Supplementary Figures 2–6. T.S.G. contributed to data in Figures 4d,f and 7b–e and Supplementary Figures 1, 2, 4 and 7. R.J.P. contributed to data in Figures 2b and Supplementary Figures 1 and 2. R.B. and R.L.J. conceived the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–3 (PDF 1603 kb)
Rights and permissions
About this article
Cite this article
Judson, R., Greve, T., Parchem, R. et al. MicroRNA-based discovery of barriers to dedifferentiation of fibroblasts to pluripotent stem cells. Nat Struct Mol Biol 20, 1227–1235 (2013). https://doi.org/10.1038/nsmb.2665
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2665
This article is cited by
-
An Insight into Reprogramming Barriers to iPSC Generation
Stem Cell Reviews and Reports (2020)
-
Arrayed functional genetic screenings in pluripotency reprogramming and differentiation
Stem Cell Research & Therapy (2019)
-
Functional screenings reveal different requirements for host microRNAs in Salmonella and Shigella infection
Nature Microbiology (2019)
-
An Insight into DNA-free Reprogramming Approaches to Generate Integration-free Induced Pluripotent Stem Cells for Prospective Biomedical Applications
Stem Cell Reviews and Reports (2019)
-
High-content screen in human pluripotent cells identifies miRNA-regulated pathways controlling pluripotency and differentiation
Stem Cell Research & Therapy (2019)