Abstract
Tuberous sclerosis complex and von Hippel–Lindau disease are distinct autosomal dominant tumor suppressor syndromes that can exhibit similar renal phenotypes and seem to share some signaling pathway components. Similarities exist in the current clinical management of, and the newly identified potential therapeutic approaches for, these conditions. This Review summarizes the pathophysiologic and therapeutic overlap between tuberous sclerosis complex and von Hippel–Lindau disease and highlights the results of recent drug trials in these settings.
Key Points
-
Tuberous sclerosis complex (TSC) and von Hippel–Lindau (VHL) disease are distinct tumor suppressor syndromes caused by mutations in the TSC1 and TSC2 genes and in the VHL gene, respectively
-
TSC and VHL disease are both associated with the development of cystic renal disease and vascular tumors, including renal cell carcinoma
-
The similarities between TSC and VHL disease are due in part to overlap in the pathways and functions of the affected genes
-
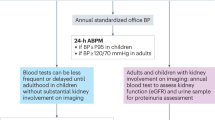
Physical examinations, imaging studies and laboratory investigations are the cornerstones of follow-up in patients with renal complications of TSC or VHL disease
-
Increased knowledge of the signaling pathways involved in TSC and VHL disease has prompted the initiation of trials to test novel pharmacological interventions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Slegtenhorst M et al. (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277: 805–808
European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75: 1305–1315
Latif F et al. (1993) Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260: 1317–1320
O'Callaghan FJ et al. (1998) Prevalence of tuberous sclerosis estimated by capture-recapture analysis. Lancet 351: 1490
Dabora SL et al. (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68: 64–80
Jones AC et al. (1999) Comprehensive mutation analysis of TSC1 and TSC2–and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 64: 1305–1315
Sancak O et al. (2005) Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype–phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet 13: 731–741
Schillinger F and Montagnac R (1996) Chronic renal failure and its treatment in tuberous sclerosis. Nephrol Dial Transplant 11: 481–485
Clarke A et al. (1999) End-stage renal failure in adults with the tuberous sclerosis complex. Nephrol Dial Transplant 14: 988–991
Shepherd CW et al. (1991) Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66: 792–796
El-Hashemite N et al. (2003) Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res 63: 5173–5177
Karbowniczek M et al. (2003) Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am J Pathol 162: 491–500
Wong AL et al. (1981) Renal angiomyolipoma: a review of the literature and a report of 4 cases. Br J Urol 53: 406–411
Obuz F et al. (2000) Various radiological appearances of angiomyolipomas in the same kidney. Eur Radiol 10: 897–899
Lin CN et al. (1994) Renal angiomyolipoma with a prominent angiomatous component and extramedullary hematopoiesis: a case report. Chin Med J 53: 185–187
Tweeddale DN et al. (1955) Angiolipoleiomyoma of the kidney: report of a case with observations on histogenesis. Cancer 8: 764–770
Mai KT et al. (1996) Epithelioid cell variant of renal angiomyolipoma. Histopathology 28: 277–280
Eble JN et al. (1997) Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol 21: 1123–1130
Farrow GM et al. (1968) Renal angiomyolipoma: a clinicopathologic study of 32 cases. Cancer 22: 564–570
Bernstein J and Robbins TO (1991) Renal involvement in tuberous sclerosis. Ann NY Acad Sci 615: 36–49
Rakowski SK et al. (2006) Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int 70: 1777–1782
Ewalt DH et al. (1998) Renal lesion growth in children with tuberous sclerosis complex. J Urol 160: 141–145
Lemaitre L et al. (1995) Renal angiomyolipoma: growth followed up with CT and/or US. Radiology 197: 598–602
Steiner MS et al. (1993) The natural history of renal angiomyolipoma. J Urol 150: 1782–1786
Kennelly MJ et al. (1994) Outcome analysis of 42 cases of renal angiomyolipoma. J Urol 152: 1988–1991
Henske EP et al. (1998) Frequent progesterone receptor immunoreactivity in tuberous sclerosis-associated renal angiomyolipomas. Mod Pathol 11: 665–668
Logginidou H et al. (2000) Frequent estrogen and progesterone receptor immunoreactivity in renal angiomyolipomas from women with pulmonary lymphangioleiomyomatosis. Chest 117: 25–30
Chesa Ponce N et al. (1995) Wunderlich's syndrome as the first manifestation of a renal angiomyolipoma [Spanish]. Arch Esp Urol 48: 305–308
Mouded IM et al. (1978) Symptomatic renal angiomyolipoma: report of 8 cases, 2 with spontaneous rupture. J Urol 119: 684–688
Kessler OJ et al. (1998) Management of renal angiomyolipoma: analysis of 15 cases. Eur Urol 33: 572–575
Zagoria RJ et al. (1991) Spontaneous perinephric hemorrhage: imaging and management. J Urol 145: 468–471
van Baal JG et al. (1994) The evolution of renal angiomyolipomas in patients with tuberous sclerosis. J Urol 152: 35–38
Koike H et al. (1994) Management of renal angiomyolipoma: a report of 14 cases and review of the literature: is nonsurgical treatment adequate for this tumor? Eur Urol 25: 183–188
Dickinson M et al. (1998) Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin Nephrol 49: 281–286
Ou YC et al. (1991) Renal angiomyolipoma: experience of 23 patients. Chin Med J 48: 217–223
Adler J et al. (1984) “Macro” aneurysm in renal angiomyolipoma: two cases, with therapeutic embolization in one patient. Urol Radiol 6: 201–203
Bissler JJ et al. (2002) Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am J Kidney Dis 39: 966–971
Yamakado K et al. (2002) Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology 225: 78–82
Pode D et al. (1985) Diagnosis and management of renal angiomyolipoma. Urology 25: 461–467
Eble JN (1998) Angiomyolipoma of kidney. Semin Diagn Pathol 15: 21–40
Bjornsson J et al. (1996) Tuberous sclerosis-associated renal cell carcinoma: clinical, pathological, and genetic features. Am J Pathol 149: 1201–1208
Hardman JA et al. (1993) Recurrent renal angiomyolipoma associated with renal carcinoma in a patient with tuberous sclerosis. Br J Urol 72: 983–984
Martignoni G et al. (2003) Renal pathology in the tuberous sclerosis complex. Pathology 35: 505–512
Ferry JA et al. (1991) Renal angiomyolipoma with sarcomatous transformation and pulmonary metastases. Am J Surg Pathol 15: 1083–1088
Lowe BA et al. (1992) Malignant transformation of angiomyolipoma. J Urol 147: 1356–1358
Folpe AL et al. (2005) Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 29: 1558–1575
Jimenez RE et al. (2001) Concurrent angiomyolipoma and renal cell neoplasia: a study of 36 cases. Mod Pathol 14: 157–163
Amin MB et al. (1997) Renal oncocytoma: a reappraisal of morphologic features with clinicopathologic findings in 80 cases. Am J Surg Pathol 21: 1–12
Eble JN and Hull MT (1984) Morphologic features of renal oncocytoma: a light and electron microscopic study. Hum Pathol 15: 1054–1061
Zerban H et al. (1987) Renal oncocytoma: origin from the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol 52: 375–387
Siracusano S et al. (1998) Rare association of renal angiomyolipoma and oncocytoma. Urology 51: 837–839
Saito K et al. (2002) Malignant clear cell “sugar” tumor of the kidney: clear cell variant of epithelioid angiomyolipoma. J Urol 168: 2533–2534
Pea M et al. (1998) Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 22: 180–187
Bonetti F et al. (1991) False-positive immunostaining of normal epithelia and carcinomas with ascites fluid preparations of antimelanoma monoclonal antibody HMB45. Am J Clin Pathol 95: 454–459
Al-Saleem T et al. (1998) Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83: 2208–2216
Robertson FM et al. (1996) Renal cell carcinoma in association with tuberous sclerosis in children. J Pediatr Surg 31: 729–730
Breysem L et al. (2002) Tuberous sclerosis with cystic renal disease and multifocal renal cell carcinoma in a baby girl. Pediatr Radiol 32: 677–680
Tello R et al. (1998) Meta analysis of the relationship between tuberous sclerosis complex and renal cell carcinoma. Eur J Radiol 27: 131–138
Argani P and Ladanyi M (2005) Translocation carcinomas of the kidney. Clin Lab Med 25: 363–378
Bernstein J and Meyer R (1967) Parenchymal maldevelopment of the kidney. In Brennemann-Kelley Practice of Pediatrics, Vol 3, 1–30 (Ed. KeHey V) New York: Harper
Potter E (1952) Pathology of the Fetus and the Newborn. Chicago: Year Book Medical Publishers
Ferrus A and Garcia-Bellido A (1976) Morphogenetic mutants detected in mitotic recombination clones. Nature 260: 425–426
Brook-Carter PT et al. (1994) Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease—a contiguous gene syndrome. Nat Genet 8: 328–332
Sampson JR and Harris PC (1994) The molecular genetics of tuberous sclerosis. Hum Mol Genet 3: 1477–1480
Patel V et al. (2008) Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590
Gullerova M and Proudfoot NJ (2008) Cohesin complex promotes transcriptional termination between convergent genes in S. pombe. Cell 132: 983–995
Martignoni G et al. (2002) Renal disease in adults with TSC2/PKD1 contiguous gene syndrome. Am J Surg Pathol 26: 198–205
Ozcan U et al. (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29: 541–551
Huang J et al. (2008) The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 28: 4104–4115
Ma L et al. (2005) Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193
Freilinger A et al. (2008) Ras mediates cell survival by regulating tuberin. Oncogene 27: 2072–2083
Lee DF et al. (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130: 440–455
Kunnimalaiyaan M et al. (2007) Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther 6: 1151–1158
Inoki K et al. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968
Tapon N et al. (2001) The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105: 345–355
Rosner M and Hengstschlager M (2004) Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2. J Biol Chem 279: 48707–48715
Rosner M et al. (2007) p27Kip1 localization depends on the tumor suppressor protein tuberin. Hum Mol Genet 16: 1541–1556
Maher ER et al. (1990) Mapping of von Hippel–Lindau disease to chromosome 3p confirmed by genetic linkage analysis. J Neurol Sci 100: 27–30
Richards FM et al. (1995) Molecular analysis of de novo germline mutations in the von Hippel–Lindau disease gene. Hum Mol Genet 4: 2139–2143
Maher ER (2004) Von Hippel–Lindau disease. Curr Mol Med 4: 833–842
Choyke PL et al. (1995) von Hippel–Lindau disease: genetic, clinical, and imaging features. Radiology 194: 629–642
Clifford SC et al. (2001) Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel–Lindau disease. Hum Mol Genet 10: 1029–1038
Binkovitz LA et al. (1990) Islet cell tumors in von Hippel–Lindau disease: increased prevalence and relationship to the multiple endocrine neoplasias. AJR Am J Roentgenol 155: 501–505
Hough DM et al. (1994) Pancreatic lesions in von Hippel–Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol 162: 1091–1094
Arao T et al. (2002) A case of von Hippel–Lindau disease with bilateral pheochromocytoma, renal cell carcinoma, pelvic tumor, spinal hemangioblastoma and primary hyperparathyroidism. Endocr J 49: 181–188
Browne G et al. (1997) Von Hippel–Lindau disease: an important differential diagnosis of polycystic kidney disease. Nephrol Dial Transplant 12: 1132–1136
Chatha RK et al. (2001) Von Hippel–Lindau disease masquerading as autosomal dominant polycystic kidney disease. Am J Kidney Dis 37: 852–858
Mandriota SJ et al. (2002) HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468
Haase VH et al. (2001) Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci USA 98: 1583–1588
Ma W et al. (2003) Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res 63: 5320–5328
Rankin EB et al. (2006) Renal cyst development in mice with conditional inactivation of the von Hippel–Lindau tumor suppressor. Cancer Res 66: 2576–2583
Jemal A et al. (2006) Cancer statistics, 2006. CA Cancer J Clin 56: 106–130
Czyzyk-Krzeska MF and Meller J (2004) von Hippel–Lindau tumor suppressor: not only HIF's executioner. Trends Mol Med 10: 146–149
Kaelin WG (2005) Proline hydroxylation and gene expression. Annu Rev Biochem 74: 115–128
Kaelin WG Jr (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2: 673–682
Gunaratnam L et al. (2003) Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(-/-) renal cell carcinoma cells. J Biol Chem 278: 44966–44974
Smith K et al. (2005) Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL−/− renal cancer. Cancer Res 65: 5221–5230
An J and Rettig MB (2005) Mechanism of von Hippel–Lindau protein-mediated suppression of nuclear factor kappa B activity. Mol Cell Biol 25: 7546–7556
Qi H and Ohh M (2003) The von Hippel–Lindau tumor suppressor protein sensitizes renal cell carcinoma cells to tumor necrosis factor-induced cytotoxicity by suppressing the nuclear factor-kappaB-dependent antiapoptotic pathway. Cancer Res 63: 7076–7080
Jermann M et al. (2006) A phase II, open-label study of gefitinib (IRESSA) in patients with locally advanced, metastatic, or relapsed renal-cell carcinoma. Cancer Chemother Pharmacol 57: 533–539
Perera AD et al. (2000) Requirement for the von Hippel–Lindau tumor suppressor gene for functional epidermal growth factor receptor blockade by monoclonal antibody C225 in renal cell carcinoma. Clin Cancer Res 6: 1518–1523
Dawson NA et al. (2004) A phase II trial of gefitinib (Iressa, ZD1839) in stage IV and recurrent renal cell carcinoma. Clin Cancer Res 10: 7812–7819
Nishimura Y et al. (2008) Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line. Mol Cancer 7: 42
Staller P et al. (2003) Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 425: 307–311
Struckmann K et al. (2008) pVHL co-ordinately regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell renal cell carcinoma. J Pathol 214: 464–471
Kuznetsova AV et al. (2003) von Hippel–Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA 100: 2706–2711
Mikhaylova O et al. (2008) The von Hippel–Lindau tumor suppressor protein and Egl-9-Type proline hydroxylases regulate the large subunit of RNA polymerase II in response to oxidative stress. Mol Cell Biol 28: 2701–2717
Galban S et al. (2003) Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Mol Cell Biol 23: 7083–7095
Roe JS et al. (2006) p53 stabilization and transactivation by a von Hippel–Lindau protein. Mol Cell 22: 395–405
Ohh M et al. (1998) The von Hippel–Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell 1: 959–968
Kurban G et al. (2006) Characterization of a von Hippel–Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res 66: 1313–1319
Hergovich A et al. (2003) Regulation of microtubule stability by the von Hippel–Lindau tumour suppressor protein pVHL. Nat Cell Biol 5: 64–70
Schermer B et al. (2006) The von Hippel–Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol 175: 547–554
Lutz MS and Burk RD (2006) Primary cilium formation requires von Hippel–Lindau gene function in renal-derived cells. Cancer Res 66: 6903–6907
Kuehn EW et al. (2007) von Hippel–Lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res 67: 4537–4540
Kamada M et al. (2001) von Hippel–Lindau protein promotes the assembly of actin and vinculin and inhibits cell motility. Cancer Res 61: 4184–4189
Esteban MA et al. (2006) Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res 66: 3567–3575
Krishnamachary B et al. (2006) Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel–Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res 66: 2725–2731
Evans AJ et al. (2007) VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol 27: 157–169
Peruzzi B et al. (2006) The von Hippel–Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci USA 103: 14531–14536
Nakaigawa N et al. (2006) Inactivation of von Hippel–Lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinoma. Cancer Res 66: 3699–3705
Koochekpour S et al. (1999) The von Hippel–Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cells. Mol Cell Biol 19: 5902–5912
Wizigmann-Voos S et al. (1995) Up-regulation of vascular endothelial growth factor and its receptors in von Hippel–Lindau disease-associated and sporadic hemangioblastomas. Cancer Res 55: 1358–1364
Brugarolas J and Kaelin WG Jr (2004) Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 6: 7–10
Inoki K et al. (2005) Dysregulation of the TSC–mTOR pathway in human disease. Nat Genet 37: 19–24
Brugarolas JB et al. (2003) TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4: 147–158
Yoder BK (2007) Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 18: 1381–1388
Praetorius HA and Spring KR (2003) The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520
Guay-Woodford LM (2003) Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034–F1049
Hou X et al. (2002) Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540
Otto EA et al. (2003) Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413–420
Yoder BK et al. (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516
Menezes LF et al. (2004) Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66: 1345–1355
Romio L et al. (2004) OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol 15: 2556–2568
Hildebrandt F and Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6: 928–940
Astrinidis A et al. (2006) Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Hum Mol Genet 15: 287–297
Shillingford JM et al. (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471
Lolkema MP et al. (2004) The von Hippel–Lindau tumor suppressor protein influences microtubule dynamics at the cell periphery. Exp Cell Res 301: 139–146
Esteban MA et al. (2006) Formation of primary cilia in the renal epithelium is regulated by the von Hippel–Lindau tumor suppressor protein. J Am Soc Nephrol 17: 1801–1806
Thoma CR et al. (2007) The VHL tumor suppressor: riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle 6: 1809–1813
Frew IJ et al. (2008) pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J 27: 1747–1757
Kugoh H et al. (2002) Retention of membrane-localized beta-catenin in cells lacking functional polycystin-1 and tuberin. Mol Carcinog 33: 131–136
Huan Y and van Adelsberg J (1999) Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest 104: 1459–1468
Serra AL et al. (2007) Clinical proof-of-concept trial to assess the therapeutic effect of sirolimus in patients with autosomal dominant polycystic kidney disease: SUISSE ADPKD study. BMC Nephrol 8: 13
Costa LJ and Drabkin HA (2007) Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist 12: 1404–1415
Bissler JJ et al. (2008) Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358: 140–151
Patel U et al. (2005) Tuberose sclerosis complex: analysis of growth rates aids differentiation of renal cell carcinoma from atypical or minimal-fat-containing angiomyolipoma. Clin Radiol 60: 665–673
Jiang X et al. (2008) The tuberous sclerosis complex regulates trafficking of glucose transporters and glucose uptake. Am J Pathol 172: 1748–1756
Acknowledgements
This work was supported by NIH Grants DK061458 and DoDTS050008 (to JJ Bissler) and NCI CA122346 and DoDPR064135 (to MF Czyzyk-Krzeska) and by the PKD Foundation (to BJ Siroky). The authors thank Jason Steinberg and Bradley Dixon for their critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Siroky, B., Czyzyk-Krzeska, M. & Bissler, J. Renal involvement in tuberous sclerosis complex and von Hippel–Lindau disease: shared disease mechanisms?. Nat Rev Nephrol 5, 143–156 (2009). https://doi.org/10.1038/ncpneph1032
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph1032
This article is cited by
-
Diagnosis and management of childhood polycystic kidney disease
Pediatric Nephrology (2011)