Key Points
-
Recent advances have improved the range of measurements that can be made using fluorescence-based single-molecule in vitro techniques, and these tools are now being used to monitor the assembly of macromolecular complexes, which has provided new mechanistic insights for the processes of DNA replication, transcription and translation in bacteria.
-
Fluorescence-based studies have revealed that the DNA polymerase holoenzyme complex contains an 'extra' core polymerase. This core polymerase, in addition to the leading-strand and lagging-strand core polymersases, can be exchanged with others from the cytosolic pool, and the complex as a whole maintains a stable association with the replication fork.
-
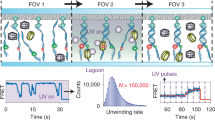
Using single-molecule studies, it has been shown that transcription initiation progresses through a well-ordered molecular pathway with a single rate-limiting step, providing a physical basis for the tight control of gene expression.
-
Single-molecule techniques have also demonstrated that throughout transcription, the bacterial RNA polymerase holoenzyme (RNAP HE)–DNA complex transitions between open and closed states, which appear to correspond to paused and processive forms of RNAP HE, respectively.
-
These technologies have also been used to investigate the dynamics of bacterial translation and have revealed that the initial stages of translation do not necessarily progress through a single series of events, but can instead follow multiple pathways.
-
In addition, a novel checkpoint has been discovered that governs the stable binding of the first non-initiator tRNA during translation initiation. This checkpoint seems to play a part in promoting a faithful transition to the elongation stage of translation.
Abstract
Decades of research have resulted in a remarkably detailed understanding of the molecular mechanisms of bacterial DNA replication, transcription and translation. Our understanding of the kinetics and physical mechanisms that drive these processes forward has been expanded by the ability of single-molecule in vitro techniques, such as force spectroscopy and single-molecule Förster (fluorescence) resonance energy transfer (smFRET), to capture short-lived intermediate states in complex pathways. Furthermore, these technologies have revealed novel mechanisms that support enzyme processivity and govern the assembly of large multicomponent complexes. Here, we summarize the application of in vitro single-molecule studies to the analysis of fundamental bacterial processes, with a focus on the most recent functional insights that have been gained from fluorescence-based methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bustamante, C., Cheng, W. & Mejia, Y. X. Revisiting the central dogma one molecule at a time. Cell 144, 480–497 (2011).
Browning, D. F. & Busby, S. J. The regulation of bacterial transcription initiation. Nature Rev. Microbiol. 2, 57–65 (2004).
Simonetti, A. et al. A structural view of translation initiation in bacteria. Cell. Mol. Life Sci. 66, 423–436 (2009).
Thanbichler, M. Synchronization of chromosome dynamics and cell division in bacteria. Cold Spring Harb. Perspect. Biol. 2, a000331 (2010).
Tinoco, I. Jr & Gonzalez, R. L. Jr . Biological mechanisms, one molecule at a time. Genes Dev. 25, 1205–1231 (2011).
Moore, P. B. How should we think about the ribosome? Annu. Rev. Biophys. 41, 1–19 (2012). A review that asks a question of increasing importance in the molecular sciences: what is the most meaningful way to describe a molecular process?
Dulin, D., Lipfert, J., Moolman, M. C. & Dekker, N. H. Studying genomic processes at the single-molecule level: introducing the tools and applications. Nature Rev. Genet. 14, 9–22 (2012). A review providing a detailed summary of the state-of-the-art tools for single-molecule experiments.
Hamdan, S. M., Loparo, J. J., Takahashi, M., Richardson, C. C. & van Oijen, A. M. Dynamics of DNA replication loops reveal temporal control of lagging-strand synthesis. Nature 457, 336–339 (2009).
Vanzi, F., Broggio, C., Sacconi, L. & Pavone, F. S. Lac repressor hinge flexibility and DNA looping: single molecule kinetics by tethered particle motion. Nucleic Acids Res. 34, 3409–3420 (2006).
van Oijen, A. M. et al. Single-molecule kinetics of λ exonuclease reveal base dependence and dynamic disorder. Science 301, 1235–1238 (2003).
Lipfert, J., Wiggin, M., Kerssemakers, J. W., Pedaci, F. & Dekker, N. H. Freely orbiting magnetic tweezers to directly monitor changes in the twist of nucleic acids. Nature Commun. 2, 439 (2011).
Chen, C. et al. Single-molecule fluorescence measurements of ribosomal translocation dynamics. Mol. Cell 42, 367–377 (2011).
Mott, M. L. & Berger, J. M. DNA replication initiation: mechanisms and regulation in bacteria. Nature Rev. Microbiol. 5, 343–354 (2007).
Duderstadt, K. E., Chuang, K. & Berger, J. M. DNA stretching by bacterial initiators promotes replication origin opening. Nature 478, 209–213 (2011).
Zorman, S., Seitz, H., Sclavi, B. & Strick, T. R. Topological characterization of the DnaA–oriC complex using single-molecule nanomanipuation. Nucleic Acids Res. 40, 7375–7383 (2012).
Tanner, N. A. et al. E. coli DNA replication in the absence of free β clamps. EMBO J. 30, 1830–1840 (2011).
Robinson, A. et al. Essential biological processes of an emerging pathogen: DNA replication, transcription, and cell division in Acinetobacter spp. Microbiol. Mol. Biol. Rev. 74, 273–297 (2010).
Robinson, A., Causer, R. J. & Dixon, N. E. Architecture and conservation of the bacterial DNA replication machinery, an underexploited drug target. Curr. Drug Targets 13, 352–372 (2012).
Soultanas, P. Loading mechanisms of ring helicases at replication origins. Mol. Microbiol. 84, 6–16 (2012).
Zhang, Z. et al. Assembly of the bacteriophage T4 primosome: single-molecule and ensemble studies. Proc. Natl Acad. Sci. USA 102, 3254–3259 (2005).
Smiley, R. D., Zhuang, Z., Benkovic, S. J. & Hammes, G. G. Single-molecule investigation of the T4 bacteriophage DNA polymerase holoenzyme: multiple pathways of holoenzyme formation. Biochemistry 45, 7990–7997 (2006).
Tanner, N. A. et al. Real-time single-molecule observation of rolling-circle DNA replication. Nucleic Acids Res. 37, e27 (2009).
Dixon, N. E. DNA replication: prime-time looping. Nature 462, 854–855 (2009).
Manosas, M., Spiering, M. M., Zhuang, Z., Benkovic, S. J. & Croquette, V. Coupling DNA unwinding activity with primer synthesis in the bacteriophage T4 primosome. Nature Chem. Biol. 5, 904–912 (2009).
Pandey, M. et al. Coordinating DNA replication by means of priming loop and differential synthesis rate. Nature 462, 940–943 (2009).
Lee, J. B. et al. DNA primase acts as a molecular brake in DNA replication. Nature 439, 621–624 (2006).
Jergic, S. et al. The unstructured C-terminus of the τ subunit of Escherichia coli DNA polymerase III holoenzyme is the site of interaction with the α subunit. Nucleic Acids Res. 35, 2813–2824 (2007).
Lia, G., Michel, B. & Allemand, J. F. Polymerase exchange during Okazaki fragment synthesis observed in living cells. Science 335, 328–331 (2012).
Loparo, J. J., Kulczyk, A. W., Richardson, C. C. & van Oijen, A. M. Simultaneous single-molecule measurements of phage T7 replisome composition and function reveal the mechanism of polymerase exchange. Proc. Natl Acad. Sci. USA 108, 3584–3589 (2011). An article describing a mechanism for core polymerase exchange at DNA replication forks, uncovered through a combination of force-based and fluorescence-based single-molecule methods.
Johnson, D. E., Takahashi, M., Hamdan, S. M., Lee, S. J. & Richardson, C. C. Exchange of DNA polymerases at the replication fork of bacteriophage T7. Proc. Natl Acad. Sci. USA 104, 5312–5317 (2007).
Reyes-Lamothe, R., Sherratt, D. J. & Leake, M. C. Stoichiometry and architecture of active DNA replication machinery in Escherichia coli. Science 328, 498–501 (2010).
McInerney, P., Johnson, A., Katz, F. & O'Donnell, M. Characterization of a triple DNA polymerase replisome. Mol. Cell 27, 527–538 (2007).
McHenry, C. S. DNA replicases from a bacterial perspective. Annu. Rev. Biochem. 80, 403–436 (2011).
Georgescu, R. E., Kurth, I. & O'Donnell, M. E. Single-molecule studies reveal the function of a third polymerase in the replisome. Nature Struct. Mol. Biol. 19, 113–116 (2012).
Gibb, B., Silverstein, T. D., Finkelstein, I. J. & Greene, E. C. Single-stranded DNA curtains for real-time single-molecule visualization of protein-nucleic acid interactions. Anal. Chem. 84, 7607–7612 (2012).
Merrikh, H., Zhang, Y., Grossman, A. D. & Wang, J. D. Replication-transcription conflicts in bacteria. Nature Rev. Microbiol. 10, 449–458 (2012).
Georgescu, R. E., Yao, N. Y. & O'Donnell, M. Single-molecule analysis of the Escherichia coli replisome and use of clamps to bypass replication barriers. FEBS Lett. 584, 2596–2605 (2010).
Herbert, K. M., Greenleaf, W. J. & Block, S. M. Single-molecule studies of RNA polymerase: motoring along. Annu. Rev. Biochem. 77, 149–176 (2008).
Larson, M. H., Landick, R. & Block, S. M. Single-molecule studies of RNA polymerase: one singular sensation, every little step it takes. Mol. Cell 41, 249–262 (2011).
Kapanidis, A. N. et al. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science 314, 1144–1147 (2006).
Revyakin, A., Liu, C., Ebright, R. H. & Strick, T. R. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science 314, 1139–1143 (2006).
Frank, J. & Gonzalez, R. L. Jr. Structure and dynamics of a processive Brownian motor: the translating ribosome. Annu. Rev. Biochem. 79, 381–412 (2010).
Yu, J. & Oster, G. A small post-translocation energy bias aids nucleotide selection in T7 RNA polymerase transcription. Biophys. J. 102, 532–541 (2012).
Friedman, L. J. & Gelles, J. Mechanism of transcription initiation at an activator-dependent promoter defined by single-molecule observation. Cell 148, 679–689 (2012). A paper describing a single-molecule approach for monitoring the entire process of transcription initiation at a bacterial promoter.
Kapanidis, A. N. et al. Retention of transcription initiation factor σ70 in transcription elongation: single-molecule analysis. Mol. Cell 20, 347–356 (2005).
Cordes, T. et al. Sensing DNA opening in transcription using quenchable Förster resonance energy transfer. Biochemistry 49, 9171–9180 (2010).
Chakraborty, A. et al. Opening and closing of the bacterial RNA polymerase clamp. Science 337, 591–595 (2012).
Sekine, S., Tagami, S. & Yokoyama, S. Structural basis of transcription by bacterial and eukaryotic RNA polymerases. Curr. Opin. Struct. Biol. 22, 110–118 (2012).
Hartzog, G. A. & Kaplan, C. D. Competing for the clamp: promoting RNA polymerase processivity and managing the transition from initiation to elongation. Mol. Cell 43, 161–163 (2011).
Tagami, S. et al. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature 468, 978–982 (2010).
Aitken, C. E., Petrov, A. & Puglisi, J. D. Single ribosome dynamics and the mechanism of translation. Annu. Rev. Biophys. 39, 491–513 (2010).
Blanchard, S. C. Single-molecule observations of ribosome function. Curr. Opin. Struct. Biol. 19, 103–109 (2009).
Laursen, B. S., Sorensen, H. P., Mortensen, K. K. & Sperling-Petersen, H. U. Initiation of protein synthesis in bacteria. Microbiol. Mol. Biol. Rev. 69, 101–123 (2005).
Milon, P. et al. The ribosome-bound initiation factor 2 recruits initiator tRNA to the 30S initiation complex. EMBO Rep. 11, 312–316 (2010).
Milon, P., Maracci, C., Filonava, L., Gualerzi, C. O. & Rodnina, M. V. Real-time assembly landscape of bacterial 30S translation initiation complex. Nature Struct. Mol. Biol. 19, 609–615 (2012).
Milon, P. & Rodnina, M. V. Kinetic control of translation initiation in bacteria. Crit. Rev. Biochem. Mol. Biol. 47, 334–348 (2012).
Tsai, A. et al. Heterogeneous pathways and timing of factor departure during translation initiation. Nature 487, 390–393 (2012). An article that describes the assembly of the bacterial translation machinery as followed by single-molecule fluorescence microscopy.
Caldas, T., Laalami, S. & Richarme, G. Chaperone properties of bacterial elongation factor EF-G and initiation factor IF2. J. Biol. Chem. 275, 855–860 (2000).
Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920 (2000).
Uemura, S. et al. Real-time tRNA transit on single translating ribosomes at codon resolution. Nature 464, 1012–1017 (2010).
Chen, C. et al. Allosteric versus spontaneous exit-site (E-site) tRNA dissociation early in protein synthesis. Proc. Natl Acad. Sci. USA 108, 16980–16985 (2011).
Karr, J. R. et al. A whole-cell computational model predicts phenotype from genotype. Cell 150, 389–401 (2012). A report that describes the first computer model of the biochemical steps that occur during the bacterial cell cycle.
Choi, P. J., Cai, L., Frieda, K. & Xie, X. S. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science 322, 442–446 (2008).
Li, G. W. & Xie, X. S. Central dogma at the single-molecule level in living cells. Nature 475, 308–315 (2011).
Taniguchi, Y. et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329, 533–538 (2010).
Levene, M. J. et al. Zero-mode waveguides for single-molecule analysis at high concentrations. Science 299, 682–686 (2003).
Loveland, A. B., Habuchi, S., Walter, J. C. & van Oijen, A. M. A general approach to break the concentration barrier in single-molecule imaging. Nature Methods 9, 987–992 (2012).
Aitken, C. E. & Puglisi, J. D. Following the intersubunit conformation of the ribosome during translation in real time. Nature Struct. Mol. Biol. 17, 793–800 (2010).
Altuntop, M. E., Ly, C. T. & Wang, Y. Single-molecule study of ribosome hierarchic dynamics at the peptidyl transferase center. Biophys. J. 99, 3002–3009 (2010).
Feldman, M. B., Terry, D. S., Altman, R. B. & Blanchard, S. C. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nature Chem. Biol. 6, 54–62 (2010).
Ly, C. T., Altuntop, M. E. & Wang, Y. Single-molecule study of viomycin's inhibition mechanism on ribosome translocation. Biochemistry 49, 9732–9738 (2010).
Munro, J. B., Wasserman, M. R., Altman, R. B., Wang, L. & Blanchard, S. C. Correlated conformational events in EF-G and the ribosome regulate translocation. Nature Struct. Mol. Biol. 17, 1470–1477 (2010).
Wang, L. et al. Allosteric control of the ribosome by small-molecule antibiotics. Nature Struct. Mol. Biol. 19, 957–963 (2012).
Nelson, S. W. & Benkovic, S. J. Response of the bacteriophage T4 replisome to noncoding lesions and regression of a stalled replication fork. J. Mol. Biol. 401, 743–756 (2010).
Yao, N. Y., Georgescu, R. E., Finkelstein, J. & O'Donnell, M. E. Single-molecule analysis reveals that the lagging strand increases replisome processivity but slows replication fork progression. Proc. Natl Acad. Sci. USA 106, 13236–13241 (2009).
Badrinarayanan, A., Reyes-Lamothe, R., Uphoff, S., Leake, M. C. & Sherratt, D. J. In vivo architecture and action of bacterial structural maintenance of chromosome proteins. Science 338, 528–531 (2012).
Lia, G. et al. RecA-promoted, RecFOR-independent progressive disassembly of replisomes stalled by helicase inactivation. Mol. Cell 49, 547–557 (2012).
Etson, C. M., Hamdan, S. M., Richardson, C. C. & van Oijen, A. M. Thioredoxin suppresses microscopic hopping of T7 DNA polymerase on duplex DNA. Proc. Natl Acad. Sci. USA 107, 1900–1905 (2010).
Sinha, N. K., Morris, C. F. & Alberts, B. M. Efficient in vitro replication of double-stranded DNA templates by a purified T4 bacteriophage replication system. J. Biol. Chem. 255, 4290–4293 (1980).
Qu, X. et al. The ribosome uses two active mechanisms to unwind messenger RNA during translation. Nature 475, 118–121 (2011).
Tang, G. Q., Roy, R., Bandwar, R. P., Ha, T. & Patel, S. S. Real-time observation of the transition from transcription initiation to elongation of the RNA polymerase. Proc. Natl Acad. Sci. USA 106, 22175–22180 (2009).
Acknowledgements
A.M.v.O. is supported by grants from the Netherlands Organisation for Scientific Research (NWO; Vici 680-47-607) and the European Research Council (ERC 281098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Derivatized glass coverslip
-
A microscope coverslip that has been coated or chemically treated to allow site-specific attachment of biomolecules.
- Flow cell
-
A microscopy sample environment that allows liquid flow over surface-immobilized particles.
- Drag
-
A force that acts on solid objects placed in a liquid flow. Drag acts in the same direction as the flow.
- Brownian motion
-
Random movement of particles in suspension, resulting from bombardment by solvent molecules.
- EMCCD camera
-
(Electron-multiplying charge-coupled device camera). A camera that can be used to capture low-level light emanating from a microscopy sample, for example.
- Fluorophore
-
A compound that is capable of producing fluorescence, such as an organic dye or a fluorescent protein.
- Förster (fluorescence) resonance energy transfer
-
A phenomenon whereby energy induced by light excitation is transferred from one fluorophore to another in a distance-dependent manner.
- Okazaki fragments
-
Regions of double-stranded DNA that are produced during discontinuous synthesis of the lagging strand.
- DNA curtain
-
A tethered-particle technique in which DNA molecules are trapped along a diffusion barrier within a flow cell and stretched parallel to the glass surface by drag.
- Rolling-circle replication
-
A mode of DNA replication of a circular substrate to yield a linear, double-stranded product of theoretically infinite length.
- Brownian ratchet
-
In the context of this article: a mechanism whereby particles undergoing Brownian motion are driven in a certain direction by selecting for only those motions that occur in the desired direction.
- Zero-mode waveguides
-
Nanostructures that are used in microscopy; in these nanostructures, light is guided through compartments that are smaller than the wavelength of the light.
- Photoswitchable fluorescent probes
-
Molecules that change from being non-fluorescent to fluorescent, or vice versa, in response to light.
Rights and permissions
About this article
Cite this article
Robinson, A., van Oijen, A. Bacterial replication, transcription and translation: mechanistic insights from single-molecule biochemical studies. Nat Rev Microbiol 11, 303–315 (2013). https://doi.org/10.1038/nrmicro2994
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2994
This article is cited by
-
Super-resolved FRET and co-tracking in pMINFLUX
Nature Photonics (2024)
-
From modeling and optimizing extraction of peels beetroot (Beta vulgaris L.) betalains to in silico probing of their antibacterial multitarget mechanisms
Biomass Conversion and Biorefinery (2023)
-
Real-time 3D single molecule tracking
Nature Communications (2020)
-
When proteins play tag: the dynamic nature of the replisome
Biophysical Reviews (2019)
-
A general approach to visualize protein binding and DNA conformation without protein labelling
Nature Communications (2016)