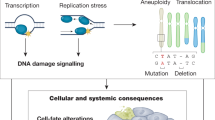
Key Points
-
Two of the best characterized progeroid syndromes are Werner syndrome and Hutchinson–Gilford progeria syndrome (HGPS). The genes targeted for mutation in these diseases (WRN and LMNA, respectively) have been identified.
-
WRN encodes a RecQ helicase and functions in homology-dependent recombination and telomere metabolism. Defective regulation of either of these fundamental nuclear processes could underlie disease.
-
LMNA encodes A-type nuclear lamins, which are targets for mutations in several human genetic diseases, known as laminopathies.
-
Specific mutations in LMNA, many of which affect farnesylation of the C terminus of lamin A, function dominantly to give rise to HGPS. Farnesylation inhibitors effectively suppress disease phenotypes in mouse models of HGPS.
-
It remains unclear whether common mechanisms underlie the progeroid syndromes and whether these mechanisms contribute significantly to the normal ageing process.
Abstract
Progeroid syndromes have been the focus of intense research in part because they might provide a window into the pathology of normal ageing. Werner syndrome and Hutchinson–Gilford progeria syndrome are two of the best characterized human progeroid diseases. Mutated genes that are associated with these syndromes have been identified, mouse models of disease have been developed, and molecular studies have implicated decreased cell proliferation and altered DNA-damage responses as common causal mechanisms in the pathogenesis of both diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Martin, G. M. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects Orig. Artic. Ser. 14, 5–39 (1978).
Friedberg, E. C. et al. DNA Repair and Mutagenesis (ASM Press, Washington, 2006).
Werner, O. in Werner's Syndrome and Human Aging (eds Salk, D., Fujiwara, Y. & Martin, G. M.) 1–14 (Plenum Press, New York, 1985).
Epstein, C. J., Martin, G. M., Schultz, A. L. & Motulsky, A. G. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 45, 177–221 (1966). Comprehensive description of clinical, pathological, cellular and genetic features of Werner syndrome.
Tollefsbol, T. O. & Cohen, H. J. Werner's syndrome: an underdiagnosed disorder resembling premature aging. Age 7, 75–88 (1984).
Goto, M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech. Ageing Dev. 98, 239–254 (1997).
Huang, S. et al. The spectrum of WRN mutations in Werner syndrome patients. Hum. Mutat. 27, 558–567 (2006).
Goto, M. Clinical characteristics of Werner syndrome and other premature aging syndromes: pattern of aging in progeroid syndromes. Gann. Monograph. Cancer Res. 49, 27–39 (2001).
Uhrhammer, N. A. et al. Werner syndrome and mutations of the WRN and LMNA genes in France. Hum. Mutat. 27, 718–719 (2006).
Yu, C. E. et al. Positional cloning of the Werner's syndrome gene. Science 272, 258–262 (1996).
Bachrati, C. Z. & Hickson, I. D. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem. J. 374, 577–606 (2003).
Opresko, P. L., Cheng, W. H. & Bohr, V. A. Junction of RecQ helicase biochemistry and human disease. J. Biol. Chem. 279, 18099–18102 (2004).
Goto, M. et al. Immunological diagnosis of Werner syndrome by down-regulated and truncated gene products. Hum. Genet. 105, 301–307 (1999).
Moser, M. J. et al. WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 28, 648–654 (2000).
Kawabe, T. et al. Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene 19, 4764–4772 (2000).
Swanson, C., Saintigny, Y., Emond, M. J. & Monnat, R. J. Jr. The Werner syndrome protein has separable recombination and survival functions. DNA Repair (Amst) 3, 475–482 (2004).
Moser, M. J. et al. Genetic instability and hematologic disease risk in Werner syndrome patients and heterozygotes. Cancer Res. 60, 2492–2496 (2000).
Ogburn, C. E. et al. An apoptosis-inducing genotoxin differentiates heterozygotic carriers for Werner helicase mutations from wild-type and homozygous mutants. Hum. Genet. 101, 121–125 (1997).
Poot, M. et al. Werner syndrome cells are sensitive to DNA cross-linking drugs. FASEB J. 15, 1224–1226 (2001).
Schellenberg, G. D., Miki, T. Yu, C. E. & Nakura, J. in The Metabolic and Molecular Basis of Inherited Disease (eds Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D.) 785–797 (McGraw-Hill, New York, 2001).
Agrelo, R. et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc. Natl Acad. Sci. USA 103, 8822–8827 (2006). Recent evidence that epigenetic silencing of WRN expression confers cellular phenotypes similar to WRN mutations, and that WRN silencing in colon cancers might have prognostic significances.
Goto, M., Miller, R. W., Ishikawa, Y. & Sugano, H. Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol. Biomarkers Prev. 5, 239–246 (1996).
Mushegian, A. R., Bassett, D. E. Jr, Boguski, M. S., Bork, P. & Koonin, E. V. Positionally cloned human disease genes: patterns of evolutionary conservation and functional motifs. Proc. Natl Acad. Sci. USA 94, 5831–5836 (1997).
Mian, I. S. Comparative sequence analysis of ribonucleases HII, III, II PH and D. Nucleic Acids Res. 25, 3187–3195 (1997).
Prince, P. R., Emond, M. J. & Monnat, R. J. Jr. Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev. 15, 933–938 (2001).
Saintigny, Y., Makienko, K., Swanson, C., Emond, M. J. & Monnat, R. J. Jr. Homologous recombination resolution defect in Werner syndrome. Mol. Cell. Biol. 22, 6971–6978 (2002). References 25 and 26 identify a role for WRN in homology-dependent recombination in human somatic cells.
Stewart, E., Chapman, C. R., Al-Khodairy, F., Carr, A. M. & Enoch, T. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16, 2682–2692 (1997).
Pichierri, P., Franchitto, A., Mosesso, P. & Palitti, F. Werner's syndrome protein is required for correct recovery after replication arrest and DNA damage induced in S-phase of cell cycle. Mol. Biol. Cell 12, 2412–2421 (2001).
Opresko, P. L. et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 14, 763–774 (2004).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 36, 877–882 (2004).
Du, X. et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell. Biol. 24, 8437–8446 (2004).
Crabbe, L., Verdun, R. E., Haggblom, C. I. & Karlseder, J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306, 1951–1953 (2004).
Laud, P. R. et al. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 19, 2560–2570 (2005).
Crabbe, L., Jauch, A., Naeger, C. M., Holtgreve-Grez, H. & Karlseder, J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl Acad. Sci. USA 104, 2205–2210 (2007).
Opresko, P. L. et al. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J. Biol. Chem. 280, 32069–32080 (2005). References 29–35 identify a role for WRN in telomere maintenance in human somatic cells and in Wrn -deficient mice.
Eller, M. S. et al. A role for WRN in telomere-based DNA damage responses. Proc. Natl Acad. Sci. USA 103, 15073–15078 (2006).
Bai, Y. & Murnane, J. P. Telomere instability in a human tumor cell line expressing a dominant-negative WRN protein. Hum. Genet. 113, 337–347 (2003).
Monnat, R. J., Jr & Saintigny, Y. Werner syndrome protein — unwinding function to explain disease. Sci. Aging Knowledge Environ. 2004, re3 (2004).
Dhillon, K. K. et al. Functional role of the Werner syndrome RecQ helicase in human fibroblasts. Aging Cell 6, 53–61 (2007).
Kipling, D., Davis, T., Ostler, E. L. & Faragher, R. G. What can progeroid syndromes tell us about human aging? Science 305, 1426–1431 (2004).
Frank, S. A. & Nowak, M. A. Problems of somatic mutation and cancer. Bioessays 26, 291–299 (2004).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Lombard, D. B. et al. Mutations in the Wrn gene in mice accelerate mortality in a p53-null background. Mol. Cell. Biol. 20, 3286–3291 (2000). Initial description of the generation and characterization of a Wrn -deficient mouse model that recapitulates the genetic and biochemical defects observed in cells from patients with WS.
Lebel, M. & Leder, P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc. Natl Acad. Sci. USA 95, 13097–13102 (1998).
Wang, L. et al. Cellular Werner phenotypes in mice expressing a putative dominant-negative human WRN gene. Genetics 154, 357–362 (2000).
Hennekam, R. C. Hutchinson–Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A 140, 2603–2624 (2006).
Hutchinson, J. Congenital absense of hair and mammary glands with atrophic condition of the skin and its appendages. Trans Med. Chir. Soc. Edinburgh 69, 473–477 (1886).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423, 293–298 (2003).
De Sandre-Giovannoli, A. et al. Lamin a truncation in Hutchinson–Gilford progeria. Science 300, 2055 (2003).
Cao, H. & Hegele, R. A. LMNA is mutated in Hutchinson–Gilford progeria (MIM 176670) but not in Wiedemann–Rautenstrauch progeroid syndrome (MIM 264090). J. Hum. Genet. 48, 271–274 (2003). References 48–50 describe the initial identification of LMNA as the gene that is mutated in HGPS.
Verstraeten, V. L. et al. Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Hum. Mol. Genet. 15, 2509–2522 (2006).
McKeon, F. D., Kirschner, M. W. & Caput, D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319, 463–468 (1986).
Stuurman, N., Heins, S. & Aebi, U. Nuclear lamins: their structure, assembly, and interactions. J. Struct. Biol. 122, 42–66 (1998).
Bridger, J. M., Kill, I. R., O'Farrell, M. & Hutchison, C. J. Internal lamin structures within G1 nuclei of human dermal fibroblasts. J. Cell Sci. 104, 297–306 (1993).
Kennedy, B. K., Barbie, D. A., Classon, M., Dyson, N. & Harlow, E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 14, 2855–2868 (2000).
Spann, T. P., Goldman, A. E., Wang, C., Huang, S. & Goldman, R. D. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J. Cell. Biol. 156, 603–608 (2002).
Ivorra, C. et al. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 20, 307–320 (2006).
Frock, R. L. et al. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 20, 486–500 (2006).
Nitta, R. T., Jameson, S. A., Kudlow, B. A., Conlan, L. A. & Kennedy, B. K. Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A-mediated cell cycle arrest. Mol. Cell. Biol. 26, 5360–5372 (2006).
Smith, E. D., Kudlow, B. A., Frock, R. L. & Kennedy, B. K. A-type nuclear lamins, progerias and other degenerative disorders. Mech. Ageing Dev. 126, 447–460 (2005).
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–920 (1999).
Nikolova, V. et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113, 357–369 (2004).
De Sandre-Giovannoli, A. et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot–Marie–Tooth disorder type 2) and mouse. Am. J. Hum. Genet. 70, 726–736 (2002).
Chen, L. et al. LMNA mutations in atypical Werner's syndrome. Lancet 362, 440–445 (2003).
Rusinol, A. E. & Sinensky, M. S. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 119, 3265–3272 (2006).
Pendas, A. M. et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nature Genet. 31, 94–99 (2002).
Dahl, K. N. et al. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 103, 10271–10276 (2006).
Scaffidi, P. & Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nature Med. 11, 440–445 (2005).
Liu, B. et al. Genomic instability in laminopathy-based premature aging. Nature Med. 11, 780–785 (2005).
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006). Evidence that the mechanisms that underlie HGPS might also have a role in normal human ageing.
Bergo, M. O. et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl Acad. Sci. USA 99, 13049–13054 (2002).
Yang, S. H. et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson–Gilford progeria syndrome mutation. J. Clin. Invest. 116, 2115–2121 (2006).
Yang, S. H. et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson–Gilford progeria syndrome mutation. Proc. Natl Acad. Sci. USA 102, 10291–10296 (2005). Reference 73, as well as 87 and 88, describe the three mouse models of HGPS that have been generated by mutating or altering Lmna expression.
Fong, L. G. et al. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311, 1621–1623 (2006). References 72 and 74 demonstrate that FTIs markedly reduce the disease phenotypes in mouse models of HGPS.
Mallampalli, M. P., Huyer, G., Bendale, P., Gelb, M. H. & Michaelis, S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 102, 14416–14421 (2005).
Glynn, M. W. & Glover, T. W. Incomplete processing of mutant lamin A in Hutchinson–Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 14, 2959–2969 (2005).
Toth, J. I. et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl Acad. Sci. USA 102, 12873–12878 (2005).
Capell, B. C. et al. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 102, 12879–12884 (2005).
Goldman, R. D. et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 101, 8963–8968 (2004).
Delbarre, E. et al. The truncated prelamin A in Hutchinson–Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum. Mol. Genet. 15, 1113–1122 (2006).
Varela, I. et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437, 564–568 (2005).
Huang, S. et al. Correction of cellular phenotypes of Hutchinson–Gilford progeria cells by RNA interference. Hum. Genet. 118, 444–450 (2005).
Csoka, A. B. et al. Genome-scale expression profiling of Hutchinson–Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell 3, 235–243 (2004).
Sephel, G. C., Sturrock, A., Giro, M. G. & Davidson, J. M. Increased elastin production by progeria skin fibroblasts is controlled by the steady-state levels of elastin mRNA. J. Invest. Dermatol. 90, 643–647 (1988).
Lemire, J. M. et al. Aggrecan expression is substantially and abnormally upregulated in Hutchinson–Gilford progeria syndrome dermal fibroblasts. Mech. Ageing Dev. 127, 660–669 (2006).
Lammerding, J. et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370–378 (2004).
Mounkes, L. C., Kozlov, S., Hernandez, L., Sullivan, T. & Stewart, C. L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, 298–301 (2003).
Varga, R. et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 103, 3250–3255 (2006).H
Fong, L. G. et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest. 116, 743–752 (2006).
Miller, R. A. 'Accelerated aging': a primrose path to insight? Aging Cell 3, 47–51 (2004).
Martin, G. M. Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120, 523–532 (2005).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
van der Pluijm, I. et al. Genome maintenance suppresses the growth hormone-insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biology 5, e2 (2007).
Monnat, R. J. Jr. From broken to old: DNA damage, IGF1endocrine suppression and aging. DNA Repair (in the press).
Kenyon, C. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 (2005).
Kamath-Loeb, A. S., Welcsh, P., Waite, M., Adman, E. T. & Loeb, L. A. The enzymatic activities of the Werner syndrome protein are disabled by the amino acid polymorphism R834C. J. Biol. Chem. 279, 55499–55505 (2004).
Davis, T., Baird, D. M., Haughton, M. F., Jones, C. J. & Kipling, D. Prevention of accelerated cell aging in Werner syndrome using a p38 mitogen-activated protein kinase inhibitor. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1386–1393 (2005).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Coppe, J. P., Kauser, K., Campisi, J. & Beausejour, C. M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 281, 29568–29574 (2006).
Csoka, A. B. et al. Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J. Med. Genet. 41, 304–308 (2004).
Plasilova, M. et al. Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson–Gilford progeria syndrome. J. Med. Genet. 41, 609–614 (2004).
Novelli, G. et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet. 71, 426–431 (2002).
Garg, A., Cogulu, O., Ozkinay, F., Onay, H. & Agarwal, A. K. A novel homozygous Ala529Val LMNA mutation in Turkish patients with mandibuloacral dysplasia. J. Clin. Endocrinol. Metab. 90, 5259–5264 (2005).
Navarro, C. L. et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum. Mol. Genet. 13, 2493–2503 (2004).
Agarwal, A. K., Fryns, J. P., Auchus, R. J. & Garg, A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, 1995–2001 (2003).
Navarro, C. L. et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum. Mol. Genet. 14, 1503–1513 (2005).
Fukuchi, K. et al. LMNA mutation in a 45 year old Japanese subject with Hutchinson–Gilford progeria syndrome. J. Med. Genet. 41, e67 (2004).
Cao, K. et al. A lamin A isoform overexpressed in Hutchinson–Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl Acad. Sci. USA 104, 4949–4954 (2007).
Dechat, T. et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl Acad. Sci. USA 104, 4955–4960 (2007).
Acknowledgements
B.A.K. was supported in part by a National Institute of General Medical Science grant. Lamin-related research in the laboratory of B.K.K. is supported by a National Institutes of Health grant. This work was supported by grants from National Cancer Institute and the Nippon Boehringer Ingelheim Virtual Research Institute on Aging to R.J.M.Jr.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
Hutchinson–Gilford progeria syndrome
FURTHER INFORMATION
Leiden Muscular Dystrophy pages
Locus-Specific Mutational Database
Glossary
- RecQ helicase
-
A protein with a domain that contains sequence and structural homology to the Escherichia coli RecQ helicase. Human RecQ helicase proteins include WRN, BLM and REQ4.
- Homology-dependent recombination
-
A potentially error-free DNA-repair pathway in which DNA double-strand breaks are repaired by limited DNA synthesis off a second, undamaged DNA molecule (for example, a sister chromatid) followed by resolution and religation of strand breaks.
- Senescence
-
A state of permanent cell-cycle exit that often occurs as a result of a cell exhausting its replicative potential. It can also result from cellular stresses or activation of oncogenes.
- Non-homologous DNA-end joining
-
A DNA-repair pathway that can operate in all phases of the cell cycle and that does not require the presence of a homologous DNA-repair template to repair DNA double-strand breaks.
- Base-excision repair
-
A DNA-repair pathway that selectively identifies and replaces single DNA bases that have been chemically damaged.
- Mesenchymal cell lineage
-
Cell lineages that are derived from the mesodermal germ layer. Includes many cell types that are found in connective tissue and other supporting, conducting or blood-forming lineages of the body.
- Nuclear lamina
-
The proteinacious meshwork that underlies the inner nuclear membrane.
- Neomorphic allele
-
An allele of a gene that confers to the encoded gene product a new activity not normally found in the product encoded by the wild-type allele.
- Hypermorphic allele
-
An allele of a gene that confers to the encoded gene product an increase in the activity that is normally associated with the product encoded by the wild-type allele.
Rights and permissions
About this article
Cite this article
Kudlow, B., Kennedy, B. & Monnat, R. Werner and Hutchinson–Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat Rev Mol Cell Biol 8, 394–404 (2007). https://doi.org/10.1038/nrm2161
Issue Date:
DOI: https://doi.org/10.1038/nrm2161
This article is cited by
-
Cellular senescence in skeletal disease: mechanisms and treatment
Cellular & Molecular Biology Letters (2023)
-
SGF29 nuclear condensates reinforce cellular aging
Cell Discovery (2023)
-
Human WRN is an intrinsic inhibitor of progerin, abnormal splicing product of lamin A
Scientific Reports (2021)
-
Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration
Cell Research (2021)
-
SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer
Protein & Cell (2020)