Key Points
-
The fibulin protein family consists of five isoforms (fibulin-1, -2, -3, -4 and -5), which are localized to the extracellular matrix.
-
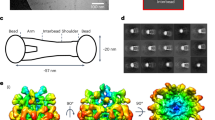
Fibulin isoforms vary in size (50–200 kDa) and have an elongated multidomain structure that is dominated by numerous calcium-binding epidermal growth factor-like modules.
-
The fibulins show a widespread deposition in various extracellular structures such as microfibrils, basement membranes and elastic fibres.
-
The widespread distribution of fibulins correlates well with their broad binding repertoire for fibronectin, collagens, basement-membrane proteins, elastin and proteoglycans.
-
The cell-biological activities of fibulins include the binding of integrin receptors and the modulation of cell proliferation and malignant transformation.
-
New information on the biological roles of the fibulins is now becoming available from the analysis of inherited human diseases and transgenic animals.
Abstract
Fibulins are a newly recognized family of extracellular matrix proteins. The five known members of the family share an elongated structure and many calcium-binding sites, owing to the presence of tandem arrays of epidermal growth factor-like domains. They have overlapping binding sites for several basement-membrane proteins, tropoelastin, fibrillin, fibronectin and proteoglycans, and they participate in diverse supramolecular structures. New insights into their biological roles are now emerging from studies of transgenic mice and of some inherited human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Argraves, W. S., Dickerson, K., Burgess, W. H. & Ruoslahti, E. Fibulin, a novel protein that interacts with the fibronectin receptor β subunit cytoplasmic domain. Cell 58, 623–629 (1989).
Argraves, W. S., Tran, H., Burgess, W. H. & Dickerson, K. Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J. Cell Biol. 111, 3155–3164 (1990). Fibulin-1 is identified and is found to be a new class of ECM protein.
Kluge, M., Mann, K., Dziadek, M. & Timpl, R. Characterization of a novel calcium-binding 90-kDa glycoprotein (BM-90) shared by basement membranes and serum. Eur. J. Biochem. 193, 651–659 (1990).
Pan, T. -C. et al. Sequence of extracellular mouse protein BM-90/fibulin and its calcium-dependent binding to other basement membrane ligands. Eur. J. Biochem. 215, 733–740 (1993).
Pan, T. -C. et al. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium-binding. J. Cell Biol. 123, 1269–1277 (1993). The first evidence that there is a fibulin protein family that contains different fibulin isoforms.
Zhang, R. -Z. et al. Fibulin-2 (FBLN2): human cDNA sequence, mRNA expression and mapping of the gene on human and mouse chromosomes. Genomics 22, 425–430 (1994).
Sasaki, T., Göhring, W., Pan, T. -C., Chu, M. -L. & Timpl, R. Binding of mouse and human fibulin-2 to extracellular matrix ligands. J. Mol. Biol. 254, 892–899 (1995).
Sasaki, T. et al. Dimer model for the microfibrillar protein fibulin-2 and identification of the connecting disulfide bridge. EMBO J. 16, 3035–3043 (1997). The first extensive model of the fibulin-2 structure.
Tran, H., Mattei, M., Godyna, S. & Argraves, W. S. Human Fibulin-1D: molecular cloning, expression and similarity with S1-5 protein, a new member of the fibulin-1 gene family. Matrix Biol. 15, 479–493 (1997).
Giltay, R., Timpl, R. & Kostka, G. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol. 18, 469–480 (1999).
Zhang, H. -Y., Lardelli, M. & Ekblom, P. Sequence of zebrafish fibulin-1 and its expression in developing heart and other embryonic organs. Dev. Genes Evol. 207, 340–351 (1997).
Barth, J. L., Argraves, K. M., Roark, E. F., Little, C. D. & Argraves, W. S. Identification of chicken and C. elegans fibulin-1 homologs and characterization of the C. elegans fibulin-1 gene. Matrix Biol. 17, 635–646 (1998).
Huber, R., Scholze, H., Paques, E. P. & Deisenhofer, J. Crystal structure analysis and molecular model of human C3a anaphylatoxin. Hoppe-Seyler's Z. Physiol. Chem. 361, 1389–1399 (1989).
Lecka-Czernik, B., Lumpkin, C. K. & Goldstein, S. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol. Cell. Biol. 15, 120–129 (1995).
Gallagher, W. M. et al. Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett. 489, 59–66 (2001).
Maurer, P. & Hohenester, E. Structural and functional aspects of calcium-binding in extracellular matrix proteins. Matrix Biol. 15, 569–580 (1997).
Downing, A. K. et al. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell 85, 597–605 (1996). This paper explains how the structure of cbEGF-like modules is stabilized by calcium ligation.
Sasaki, T., Mann, K., Murphy, G., Chu, M. -L. & Timpl, R. Different susceptibilities of fibulin-1 and fibulin-2 to cleavage by matrix metalloproteinases and other tissue proteases. Eur. J. Biochem. 240, 427–434 (1996).
Sasaki, T. et al. Structural characterization of two variants of fibulin-1 that differ in nidogen affinity. J. Mol. Biol. 245, 241–250 (1995).
Roark, E. F. et al. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J. Histochem. Cytochem. 43, 401–411 (1995).
Stone, E. M. et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nature Genet. 22, 199–202 (1999).
Nakamura, T. et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415, 171–175 (2002).
Yanagisawa, H. et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415, 168–171 (2002). This work describes, together with reference 22, the role of fibulin-5 in stabilizing elastic fibres.
Spence, S. G., Argraves, W. S., Walters, L., Hungerford, J. E. & Little, C. Fibulin is localized at sites of epithelial–mesenchymal transitions in the early avian embryo. Dev. Biol. 151, 473–484 (1992).
Zhang, H. -Y., Timpl, R., Sasaki, T., Chu, M. -L. & Ekblom, P. Fibulin-1 and fibulin-2 expression during organogenesis in the developing mouse embryo. Dev. Dyn. 205, 348–364 (1996).
Miosge, N. et al. The extracellular matrix proteins fibulin-1 and fibulin-2 in the early human embryo. Histochem. J. 28, 109–116 (1996).
Hungerford, J. E., Owens, G. K., Argraves, W. S. & Little, C. D. Development of aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev. Biol. 178, 375–392 (1996).
Bouchey, D., Argraves, W. S. & Little, C. D. Fibulin-1, vitronectin expression during avian cardiac valve and septa development. Anat. Rec. 244, 540–551 (1996).
Zhang, H. -Y., Kluge, M., Timpl, R., Chu, M. -L. & Ekblom, P. The extracellular matrix glycoproteins BM-90 and tenascin are expressed in the mesenchyme at sites of endothelial–mesenchymal conversion in the embryonic mouse heart. Differentiation 52, 211–220 (1993).
Zhang, H. -Y. et al. Extracellular matrix protein fibulin-2 is expressed in the embryonic endocardial cushion tissue and is a prominent component of valves in adult heart. Dev. Biol. 167, 18–26 (1995).
Tsuda, T., Wang, H., Timpl, R. & Chu, M. -L. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels and coronary vessels. Dev. Dyn. 222, 89–100 (2001).
Miosge, N., Sasaki, T., Chu, M. -L., Herken, R. & Timpl, R. Ultrastructural localization of microfibrillar fibulin-1 and fibulin-2 during heart development indicates a switch in molecular associations. Cell. Mol. Life Sci. 54, 606–613 (1997).
Gallagher, W. M. et al. MBP1: a novel mutant p53-specific protein partner with oncogenic properties. Oncogene 18, 3608–3616 (1999).
Kowal, R. C., Richardson, J. A., Miano, J. M. & Olsen, E. N. EVEC. A novel epidermal growth factor-like repeat containing protein upregulated in embryonic and injured adult vasulature. Circ. Res. 84, 1166–1176 (1999).
Ohsawa, I., Takamura, C. & Kohsaka, S. Fibulin-1 binds the amino-terminal head of β-amyloid precursor protein. J. Neurochem. 76, 1411–1420 (2001).
Reinhardt, D. P. et al. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem. 271, 19489–19496 (1996).
Sasaki, T. et al. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 460, 280–284 (1999).
Sasaki, T., Wiedemann, H., Matzner, M., Chu, M. -L. & Timpl, R. Expression of fibulin-2 by fibroblasts and deposition with fibronectin into a fibrillar matrix. J. Cell Sci. 109, 2895–2904 (1996).
Loveland, K. et al. Developmental changes in the basement membrane of the normal and hypothyroid postnatal rat testis: segmental localization of fibulin-2 and fibronectin. Biol. Reprod. 58, 1123–1130 (1998).
Raghunath, M. et al. Confocal laser scanning analysis of the association of fibulin-2 with fibrillin-1 and fibronectin define different stages of skin regeneration. J. Invest. Dermatol. 112, 97–101 (1999).
Fässler, R., Sasaki, T., Timpl, R., Chu, M. -L. & Werner, S. Differential regulation of fibulin, tenascin and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp. Cell Res. 222, 111–116 (1996).
Hunzelmann, N., Nischt, R., Brenneisen, P., Eickert, A. & Krieg, T. Increased deposition of fibulin-2 in solar elastosis and its colocalization with elastic fibers. Brit. J. Dermatol. 145, 217–222 (2001).
Kusubata, M. et al. Spatiotemporal changes of fibronectin, tenascin-C, fibulin-1, and fibulin-2 in the skin during the development of chronic contact dermatitis. J. Invest. Dermatol. 113, 906–912 (1999).
Roger, P., Pujol, P., Lucas, A., Baldet, P. & Rochefort, H. Increased immunostaining of fibulin-1, an estrogen-regulated protein in the stroma of human ovarian epithelial tumors. Am. J. Pathol. 153, 1579–1588 (1998).
Balbona, K. et al. Fibulin binds to itself and to the carboxyl-terminal heparin-binding region of fibronectin. J. Biol. Chem. 267, 20120–20125 (1992).
Tran, H., Van Dusen, W. J. & Argraves, W. S. The self-association and fibronectin-binding sites of fibulin-1 map to calcium-binding epidermal growth factor-like domains. J. Biol. Chem. 272, 22600–22606 (1997).
Roman, J. & McDonald, J. A. Fibulin's organization into the extracellular matrix of fetal lung fibroblasts is dependent on fibronectin matrix assembly. Am. J. Respir. Cell Mol. Biol. 8, 538–545 (1993).
Godyna, S., Mann, D. M. & Argraves, W. S. A quantitative analysis of the incorporation of fibulin-1 into the extracellular matrix indicates that fibronectin assembly is required. Matrix Biol. 14, 467–477 (1994).
Adam, S. et al. Binding of fibulin-1 to nidogen depends on its C-terminal globular domain and a specific array of calcium-binding epidermal growth factor-like (EG) modules. J. Mol. Biol. 272, 226–236 (1997).
Ries, A., Göhring, W., Fox, J. W., Timpl, R. & Sasaki, T. Recombinant domains of mouse nidogen-1 and their binding to basement membrane proteins and monoclonal antibodies. Eur. J. Biochem. 268, 5119–5128 (2001).
Friedrich, M. V. K. et al. Structural basis of glycosaminoglycan modification and of heterotypic interactions of perlecan domain V. J. Mol. Biol. 294, 259–270 (1999).
Hopf, M., Göhring, W., Mann, K. & Timpl, R. Mapping of binding sites for nidogens, fibulin-2, fibronectin and heparin to different IG modules of perlecan. J. Mol. Biol. 311, 529–541 (2001).
Timpl, R. et al. Structure and function of laminin LG modules. Matrix Biol. 19, 309–317 (2000).
Talts, J. F., Andac, Z., Göhring, W., Brancaccio, A. & Timpl, R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 18, 863–870 (1999).
Utani, A., Nomizu, M. & Yamada, Y. Fibulin-2 binds to the short arms of laminin-5 and laminin-1 via conserved amino acid sequences. J. Biol. Chem. 272, 2814–2820 (1997).
Sasaki, T. et al. Short arm region of laminin-5 γ-2 chain: structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 314, 751–763 (2001).
Sasaki, T. et al. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 17, 4249–4256 (1998).
Sasaki, T. et al. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 301, 1179–1190 (2000).
Sasaki, T., Hohenester, E. & Timpl, R. Structure and function of collagen-derived endostatin inhibitors of angiogenesis. IUBMB Life 53, 77–84 (2002).
Ruoslahti, E. Brain extracellular matrix. Glycobiology 6, 489–492 (1996).
Aspberg, A., Adam, S., Kostka, G., Timpl, R. & Heinegard, D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J. Biol. Chem. 274, 20444–20449 (1999).
Olin, A. J. et al. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding. J. Biol. Chem. 276, 1253–1261 (2001).
Tran, H. et al. The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J. Biol. Chem. 270, 19458–19464 (1995).
Godyna, S., Dias-Ricart, M. & Argraves, W. S. Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood 88, 2569–2577 (1996).
Kostka, G. et al. Perinatal lethality and endothedlial cell abnormalities in several vessel compartments of fibulin-1 deficient mice. Mol. Cell. Biol. 21, 7025–7034 (2001). This paper shows that a fibulin-1 deficiency causes a haemorrhagic phenotype.
Perbal, B. et al. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin-1C: a clue for a role of NOVH in cell-adhesion signalling. Proc. Natl Acad. Sci. USA 96, 869–874 (1999).
Pfaff, M., Sasaki, T., Tangemann, K., Chu, M. -L. & Timpl, R. Integrin-binding and cell-adhesion studies of fibulins reveal a particular affinity for αIIbβ3. Exp. Cell Res. 219, 87–92 (1995).
Twal, W. O. et al. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J. Cell Sci. 114, 4587–4598 (2001).
Nakamura, T. et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 274, 22476–22483 (1999).
Schiemann, W. P., Blobe, G. C., Kalume, D. E., Pandey, A. & Lodish, H. F. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-β and affects protein kinase cascades. J. Biol. Chem. 277, 27367–27377 (2002).
Heine, H., Delude, R. L., Monks, B. G., Esperik, T. & Golenbock, D. T. Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J. Biol. Chem. 274, 21049–21055 (1999).
Qing, J. et al. Surpression of anchorage-independent growth and matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene 15, 2159–2168 (1997).
Clinton, G. M. et al. Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. Proc. Natl Acad Sci. USA 93, 316–320 (1996).
Hayashido, Y. et al. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int. J. Cancer 75, 654–658 (1998).
Debeer, P. et al. The fibulin-1 gene (FLBN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet. 39, 98–104 (2002).
Tartellin, E. E. et al. Molecular genetic heterogeneity in autosomal dominant drusen. J. Med. Genet. 38, 381–384 (2001).
Marmorstein, L. Y. et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc. Natl Acad. Sci. USA 99, 13067–13072 (2002).
Midwood, K. S. & Schwarzbauer, J. E. Elastic fibres: building bridges between cells and their matrix. Curr. Biol. 12, R279–R281 (2002).
Loeys, B. et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11, 2113–2118 (2002).
Markova, D. et al. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 72, 998–1004 (2003).
Kielty, C. M., Sherrat, M. J. & Shuttleworth, C. A. Elastic fibers. J. Cell Sci. 115, 2817–2828 (2002).
Dietz, H. C. & Mecham, R. P. Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol. 19, 481–488 (2000).
Milewicz, D. M., Urban, Z. & Boyd, C. Genetic disorders of the elastic fiber system. Matrix Biol. 19, 471–480 (2000).
Pan, T. -C., Kostka, G., Zhang, R. -Z., Timpl, R. & Chu, M. -L. Complete exon–intron organization of the mouse fibulin-1 gene and its comparison with the human fibulin-1 gene. FEBS Lett. 444, 38–42 (1999).
Mattei, M. G., Pan, T. -C., Zhang, R. -Z., Timpl, R. & Chu, M. -L. The fibulin-1 gene (FBLN1) is located on human chromosome 22 and mouse chromosome 15. Genomics 22, 437–438 (1994).
Grässel, S., Sicot, F. -X., Gotta, S. & Chu, M. -L. Mouse fibulin-2 gene. Complete exon–intron organization and promoter characterization. Eur. J. Biochem. 263, 471–477 (1999).
Ikegawa, S., Toda, T., Okui, K. & Nakamura, Y. Structure and chromosomal assignment of the human S1-5 gene (FBNL) that is highly homologous to fibrillin. Genomics 35, 590–592 (1996).
Katsanis, N., Venable, S., Smith, J. R. & Lupski, J. R. Isolation of a paralog of the Doyne honeycombe retinal dystrophy gene from the multiple retinopathy critical region on 11q13. Hum. Genet. 106, 66–72 (2000).
Kowal, R. C., Jolsin, J. M., Olson, E. N. & Schultz, R. A. Assignment of fibulin–5 (FBLN5) to human chromosome 14q31 by in situ hybridization and radiation hybrid mapping. Cytogenet. Cell Genet. 87, 2–3 (1999).
Acknowledgements
Part of the work described here was supported by research grants from the Deutsche Forschungsgemeinschaft for T.S. and R.T., the European Community for R.T., and the National Institutes of Health for M.-L.C. The authors are grateful to S. Argraves for communicating unpublished work and to J. Uitto for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
InterPro
LocusLink
Swiss-Prot
FURTHER INFORMATION
Glossary
- EDTA
-
(ethylenediamine tetra-acetic acid). A strong chelator of bivalent cations.
- Kd VALUE
-
The equilibrium dissociation constant of bimolecular reactions.
- PERINEURAL TISSUE
-
The tissue around a nerve or group of nerves.
- ELASTOTIC SKIN
-
Skin that shows degenerative changes of the elastic fibres. The condition can be associated with skin diseases, ageing or prolonged exposure to sunlight.
- CONTACT DERMATITIS
-
Itching, redness or inflammation of the skin that is caused by direct exposure to irritating substances, such as chemicals, metals, clothing, cosmetics and plants.
- ELASTINOPATHY
-
Pathological changes of the elastic fibres that lead to various degenerative diseases.
- MESENCHYMAL TISSUE
-
The tissue that originates from mesenchymal cells, which are unspecified cells that are derived mainly from the primitive mesoderm during embryogenesis. The connective tissues of the body develop from the mesenchymal cells.
- ANGIOGENESIS
-
A process of blood-vessel branching, in which blood vessels sprout from small capillaries.
- RGD-DEPENDENT INTEGRINS
-
A specific group of cellular integrin receptors that bind to the arginine-glycine-aspartate (RGD) sequences of their ligands.
- WERNER SYNDROME
-
A premature ageing disorder that is inherited in an autosomal recessive mode. The clinical symptoms can include short stature, wrinkled skin, baldness, cataracts and muscular atrophy.
- SYNPOLYDACTYLY
-
A developmental defect that is characterized by the fusion (syndactyly) and splitting (polydactyly) of fingers or toes. It is usually an autosomal dominant disease and can result from mutations in the homeobox genes.
- MACULAR DEGENERATIVE DISEASE
-
An incurable eye disease that is caused by the deterioration of the central portion of the retina, which is known as the macula. The disease is heterogeneous and includes the rare heritable forms, the sporadic early-onset form and the age-related form.
- MARFAN SYNDROME
-
A heritable connective tissue disease that affects several organ systems, including the skeleton, eyes, lungs, heart and blood vessels. The disease is caused by dominant mutations in the fibrillin-1 gene.
Rights and permissions
About this article
Cite this article
Timpl, R., Sasaki, T., Kostka, G. et al. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4, 479–489 (2003). https://doi.org/10.1038/nrm1130
Issue Date:
DOI: https://doi.org/10.1038/nrm1130
This article is cited by
-
Genetic screening for anticancer genes highlights FBLN5 as a synthetic lethal partner of MYC
Cell Communication and Signaling (2023)
-
Unveiling the role of osteosarcoma-derived secretome in premetastatic lung remodelling
Journal of Experimental & Clinical Cancer Research (2023)
-
EFEMP2 upregulates PD-L1 expression via EGFR/ERK1/2/c-Jun signaling to promote the invasion of ovarian cancer cells
Cellular & Molecular Biology Letters (2023)
-
Tubulointerstitial nephritis antigen-like 1 is a novel matricellular protein that promotes gastric bacterial colonization and gastritis in the setting of Helicobacter pylori infection
Cellular & Molecular Immunology (2023)
-
Serum proteome profiling of naturally acquired Babesia rossi infection in dogs
Scientific Reports (2023)