Key Points
-
Gap junctions are aggregates of intercellular channels that join adjacent cells. Intercellular channels are composed of a pair of connexons, each of which is a hexamer of connexin proteins. As precursors to the intercellular channels, connexons can be found in the plasma membranes of cells. In some cellular systems, however, connexons might function independently of gap junctions.
-
Although there are data implicating gap-junctional intercellular channels in the cell–cell transmission of Ca2+ waves, it is also clear that these waves can spread by paracrine signalling owing to the extracellular release of ATP that binds to purinergic receptors. Evidence is accumulating that the connexon might provide a regulated channel that is responsible for the ATP release.
-
The bisphosphonate alendronate has been shown to have an anti-apoptotic effect on bone cells. Recent data indicate that connexons might be the receptor for alendronate, transducing a survival signal through the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway.
-
A long-standing puzzle has been the interaction of retinal horizontal cells with cones as part of centre-surround antagonism. The interaction between these cells might involve connexons. The voltage-induced opening of connexons in the horizontal-cell membrane might function to create a negative extracellular potential in the invaginating synapses of the cone pedicles, opening Ca2+ channels and stimulating glutamate release.
Abstract
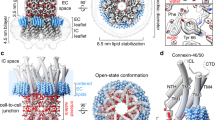
Gap junctions consist of intercellular channels that connect the cytoplasm of adjacent cells directly and allow the exchange of small molecules. These channels are unique in that they span two plasma membranes — the more orthodox ion or ligand-gated channels span only one. Each cell contributes half of the intercellular channel, and each half is known as a connexon or hemichannel. Recent studies indicate that connexons are also active in single plasma membranes and that they might be essential in intercellular signalling beyond their incorporation into gap junctions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fraser, S. E., Green, C. R., Bode, H. R. & Gilula, N. B. Selective disruption of gap junctional communication interferes with a patterning process in hydra. Science 237, 49–55 (1987).
Deans, M. R., Volgyi, B., Goodenough, D. A., Bloomfield, S. A. & Paul, D. L. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36, 703–712 (2002).
Valiunas, V., Bukauskas, F. F. & Weingart, R. Conductances and selective permeability of connexin43 gap junction channels examined in neonatal rat heart cells. Circ. Res. 80, 708–719 (1997).
Lampe, P. D. & Lau, A. F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 384, 205–215 (2000).
Harris, A. L. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472 (2001).
Veenstra, R. D. et al. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ. Res. 77, 1156–1165 (1995).
Elfgang, C. et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 129, 805–817 (1995).
Cao, F. L. et al. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J. Cell Sci. 111, 31–43 (1998).
Bevans, C. G., Kordel, M., Rhee, S. K. & Harris, A. L. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 273, 2808–2816 (1998).
He, D. S., Jiang, J. X., Taffet, S. M. & Burt, J. M. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc. Natl Acad. Sci. USA 96, 6495–6500 (1999).
White, T. W., Bruzzone, R., Wolfram, S., Paul, D. L. & Goodenough, D. A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J. Cell Biol. 125, 879–892 (1994).
Cottrell, G. T. & Burt, J. M. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am. J. Physiol. Cell Physiol. 281, C1559–C1567 (2001).
Paul, D. L., Ebihara, L., Takemoto, L. J., Swenson, K. I. & Goodenough, D. A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115, 1077–1089 (1991).
Musil, L. S. & Goodenough, D. A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 115, 1357–1374 (1991). This paper presents biochemical evidence of connexons on the cell surface before incorporation into gap-junctional intercellular channels.
Musil, L. S. & Goodenough, D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74, 1065–1077 (1993).
DeVries, S. H. & Schwartz, E. A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. (Lond.) 445, 201–230 (1992). This is a classic and beautiful functional demonstration of open connexons in the plasma membrane of a single cell.
Bennett, M. V. L. & Goodenough, D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci. Res. Program Bull. 16, 373–486 (1978).
Nelles, E. et al. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl Acad. Sci. USA 93, 9565–9570 (1996).
Simon, A. M., Goodenough, D. A., Li, E. & Paul, D. L. Female infertility in mice lacking connexin 37. Nature 385, 525–529 (1997).
Gong, X. et al. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843 (1997).
White, T. W., Goodenough, D. A. & Paul, D. L. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 143, 815–825 (1998).
Bergoffen, J. et al. Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science 262, 2039–2042 (1993).
Richard, G. Connexins: a connection with the skin. Exp. Dermatol. 9, 77–96 (2000).
Berry, V. et al. Connexin 50 mutation in a family with congenital 'zonular nuclear' pulverulent cataract of Pakistani origin. Hum. Genet. 105, 168–170 (1999).
Mackay, D. et al. Connexin46 mutations in autosomal dominant congenital cataract. Am. J. Hum. Genet. 64, 1357–1364 (1999).
Polyakov, A., Shagina, I., Khlebnikova, O. & Evgrafov, O. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin. Genet. 60, 476–478 (2001).
Shiels, A. et al. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant zonular pulverulent cataract, on chromosome 1q. Am. J. Hum. Genet. 62, 526–532 (1998).
Kelsell, D. P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 (1997).
Willecke, K. et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737 (2002).
Rabionet, R., Lopez-Bigas, N., Arbones, M. L. & Estivill, X. Connexin mutations in hearing loss, dermatological and neurological disorders. Trends Mol. Med. 8, 205–212 (2002).
Richard, G. Connexin disorders of the skin. Adv. Dermatol. 17, 243–277 (2001).
White, T. W. & Paul, D. L. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol. 61, 283–310 (1999).
Meyer, R. A., Laird, D. W., Revel, J. P. & Johnson, R. G. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J. Cell Biol. 119, 179–189 (1992).
Goodenough, D. A. & Revel, J. P. The permeability of isolated and in situ mouse hepatic gap junctions studied with enzymatic tracers. J. Cell Biol. 50, 81–91 (1971).
Buehler, L. K., Stauffer, K. A., Gilula, N. B. & Kumar, N. M. Single channel behavior of recombinant β2 gap junction connexons reconstituted into planar lipid bilayers. Biophys. J. 68, 1767–1775 (1995).
Ebihara, L. & Steiner, E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 102, 59–74 (1993).
DeVries, S. H. & Schwartz, E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J. Physiol 414, 351–375 (1989).
Li, H. Y. et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 134, 1019–1030 (1996).
Hofer, A. & Dermietzel, R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia 24, 141–154 (1998).
Kondo, R. P., Wang, S. Y., John, S. A., Weiss, J. N. & Goldhaber, J. I. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J. Mol. Cell Cardiol. 32, 1859–1872 (2000).
Beahm, D. L. & Hall, J. E. Hemichannel and junctional properties of connexin 50. Biophys. J. 82, 2016–2031 (2002).
Valiunas, V. Biophysical properties of Connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 119, 147–164 (2002).
White, T. W. et al. Functional characteristics of skate connexin35, a member of the γ subfamily of connexins expressed in the vertebrate retina. Eur. J. Neurosci. 11, 1883–1890 (1999).
Arellano, R. O., Woodward, R. M. & Miledi, R. A monovalent cationic conductance that is blocked by extracellular divalent cations in Xenopus oocytes. J. Physiol. (Lond.) 484, 593–604 (1995).
Ebihara, L. Xenopus connexin38 forms hemi-gap junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys. J. 71, 742–748 (1996).
Quist, A. P., Rhee, S. K., Lin, H. & Lal, R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 148, 1063–1074 (2000).
Turin, L. & Warner, A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryos. Nature 270, 56–57 (1977).
Trexler, E. B., Bukauskas, F. F., Bennett, M. V., Bargiello, T. A. & Verselis, V. K. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 113, 721–742 (1999).
Ebihara, L., Berthoud, V. M. & Beyer, E. C. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys. J. 68, 1796–1803 (1995).
Verselis, V. K., Trexler, E. B. & Bukauskas, F. F. Connexin hemichannels and cell–cell channels: comparison of properties. Braz. J. Med. Biol. Res. 33, 379–389 (2000).
Eskandari, S., Zampighi, G. A., Leung, D. W., Wright, E. M. & Loo, D. D. Inhibition of gap junction hemichannels by chloride channel blockers. J. Membr. Biol. 185, 93–102 (2002).
Zhang, Y., McBride, D. W. & Hamill, O. P. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. J. Physiol. (Lond.) 508, 763–776 (1998).
Jordan, K., Chodock, R., Hand, A. R. & Laird, D. W. The origin of annular junctions: a mechanism of gap junction internalization. J. Cell Sci. 114, 763–773 (2001).
Jordan, K. et al. Trafficking, assembly, and function of a connexin43–green fluorescent protein chimera in live mammalian cells. Mol. Biol. Cell 10, 2033–2050 (1999).
Kumar, N. M., Friend, D. S. & Gilula, N. B. Synthesis and assembly of human β1 gap junctions in BHK cells by DNA transfection with the human β1 cDNA. J. Cell Sci. 108, 3725–3734 (1995).
Falk, M. M. Connexin-specific distribution within gap junctions revealed in living cells. J. Cell Sci. 113, 4109–4120 (2000).
Lauf, U. et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl Acad. Sci. USA 99, 10446–10451 (2002).
Contreras, J. E. et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl Acad. Sci. USA 99, 495–500 (2002).
John, S. A., Kondo, R., Wang, S. Y., Goldhaber, J. I. & Weiss, J. N. Connexin-43 hemichannels opened by metabolic inhibition. J. Biol. Chem. 274, 236–240 (1999).
Li, F., Sugishita, K., Su, Z., Ueda, I. & Barry, W. H. Activation of connexin-43 hemichannels can elevate [Ca2+]i and [Na+]i in rabbit ventricular myocytes during metabolic inhibition. J. Mol. Cell. Cardiol. 33, 2145–2155 (2001).
Cotrina, M. L. et al. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl Acad. Sci. USA 95, 15735–15740 (1998).
Charles, A. C. et al. Intercellular calcium signaling via gap junctions in glioma cells. J. Cell Biol. 118, 195–201 (1992).
Finkbeiner, S. Calcium waves in astrocytes — filling in the gaps. Neuron 8, 1101–1108 (1992).
Venance, L., Stella, N., Glowinski, J. & Giaume, C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J. Neurosci. 17, 1981–1992 (1997).
D'Andrea, P. et al. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology 37, 75–83 (2000).
Frame, M. K. & de Feijter, A. W. Propagation of mechanically induced intercellular calcium waves via gap junctions and ATP receptors in rat liver epithelial cells. Exp. Cell Res. 230, 197–207 (1997).
Sanderson, M. J., Charles, A. C. & Dirksen, E. R. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1, 585–596 (1990).
Guthrie, P. B. et al. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528 (1999).
Cotrina, M. L., Lin, J. H., Lopez-Garcia, J. C., Naus, C. C. & Nedergaard, M. ATP-mediated glia signaling. J. Neurosci. 20, 2835–2844 (2000).
Jorgensen, N. R. et al. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 277, 7574–7580 (2002).
Jorgensen, N. R. et al. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J. Bone Miner. Res. 15, 1024–1032 (2000).
Fry, T., Evans, J. H. & Sanderson, M. J. Propagation of intercellular calcium waves in C6 glioma cells transfected with connexins 43 or 32. Microsc. Res. Tech. 52, 289–300 (2001). This state-of-the-art study shows that Cx43- and Cx32-based gap junctions are permeable to InsP 3 and that Ca2+ wave propagation through gap junctions is dependent on the diffusion of InsP3 but not Ca2+.
Suadicani, S. O., Vink, M. J. & Spray, D. C. Slow intercellular Ca2+ signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. Am. J. Physiol Heart Circ. Physiol. 279, H3076–H3088 (2000).
Scemes, E., Suadicani, S. O. & Spray, D. C. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J. Neurosci. 20, 1435–1445 (2000).
Klepeis, V. E., Cornell-Bell, A. & Trinkaus-Randall, V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J. Cell Sci. 114, 4185–4195 (2001).
Braet, K., Vandamme, W., Martin, P. E., Evans, W. H. & Leybaert, L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33, 37–48 (2003). Here, synthetic peptides that correspond to the extracellular sequences of connexins were used in studies designed to interfere with the function of connexons.
Blomstrand, F., Aberg, N. D., Eriksson, P. S., Hansson, E. & Ronnback, L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience 92, 255–265 (1999).
Stout, C. E., Costantin, J. L., Naus, C. C. & Charles, A. C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. (2002). This paper shows that both propagation of Ca2+ waves and the release of ATP from astrocytes are repressed by the gap-junction blocker FFA.
Guan, X. et al. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 139, 1785–1792 (1997).
Lee, H. C., Galione, A. & Walseth, T. F. Cyclic ADP-ribose: metabolism and calcium mobilizing function. Vitam. Horm. 48, 199–257 (1994).
Zocchi, E. et al. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: role of NAD+ transport across cell membranes. FASEB J. 13, 273–283 (1999).
Bruzzone, S., Guida, L., Zocchi, E., Franco, L. & De Flora, A. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15, 10–12 (2001). These experiments revealed a temperature-independent NAD+ transport system in the plasma membrane of 3T3 fibroblast cells, which could be blocked by 18 α-glycyrrhetinic acid or by treatment with antisense-Cx43 oligonucleotides.
Franco, L. et al. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J. Biol. Chem. 276, 48300–48308 (2001).
Verderio, C. et al. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J. Neurochem. 78, 646–657 (2001).
Bruzzone, S. et al. A self-restricted CD38-connexin 43 cross-talk affects NAD+ and cyclic ADP-ribose metabolism and regulates intracellular calcium in 3T3 fibroblasts. J. Biol. Chem. 276, 48300–48308 (2001).
Zocchi, E. et al. Expression of CD38 increases intracellular calcium concentration and reduces doubling time in HeLa and 3T3 cells. J. Biol. Chem. 273, 8017–8024 (1998).
Schiller, P. C., Mehta, P. P., Roos, B. A. & Howard, G. A. Hormonal regulation of intercellular communication: parathyroid hormone increases connexin 43 gene expression and gap-junctional communication in osteoblastic cells. Mol. Endocrinol. 6, 1433–1440 (1992).
Steinberg, T. H. et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 13, 744–750 (1994).
Ilvesaro, J., Vaananen, K. & Tuukkanen, J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J. Bone Miner. Res. 15, 919–926 (2000).
Reaume, A. G. et al. Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834 (1995).
Lecanda, F. et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 151, 931–944 (2000).
Orcel, P. & Beaudreuil, J. Bisphosphonates in bone diseases other than osteoporosis. Joint Bone Spine 69, 19–27 (2002).
Weinstein, R. S. & Manolagas, S. C. Apoptosis and osteoporosis. Am. J. Med. 108, 153–164 (2000).
Halasy-Nagy, J. M., Rodan, G. A. & Reszka, A. A. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29, 553–559 (2001).
Plotkin, L. I. et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 104, 1363–1374 (1999).
Plotkin, L. I., Manolagas, S. C. & Bellido, T. Transduction of cell survival signals by connexin 43 hemichannels. J. Biol. Chem. 277, 8648–8657 (2002). Inhibition of apoptosis is seen in bone cells that express Cx43, but not in those derived from the cx43 -knockout mouse, which indicates a requirement for connexin expression. In addition, the ability to inhibit apoptosis can be restored to cx43−/− cells by transfection with Cx43.
Plotkin, L. I. & Bellido, T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of connexin43. Cell Adhes. Commun. 8, 377–382 (2001).
Giepmans, B. N., Hengeveld, T., Postma, F. R. & Moolenaar, W. H. Interaction of c-Src with gap junction protein connexin-43: role in the regulation of cell–cell communication. J. Biol. Chem. 276, 8544–8549 (2000). The carboxy-terminal cytoplasmic domain of Cx43 interacts directly with, and is phosphorylated by, Src, and this results in inhibition of gap junctional intercellular channels, and by implication, connexons.
Boucher, M. J. et al. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell Biochem. 79, 355–369 (2000).
Yuan, P. X. et al. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 276, 31674–31683 (2001).
Huang, R. P. et al. Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human glioblastoma cells. Int. J. Cancer 92, 130–138 (2001).
Kuffler, S. W. Discharge patterns and functional organization of the mammalian retina. J. Neurophysiol. 16, 37–68 (1953).
Barlow, H. B. & Levick, W. R. The mechanism of directionally selective units in rabbit's retina. J. Physiol 178, 477–504 (1965).
Werblin, F. S. & Dowling, J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 32, 339–355 (1969).
Baylor, D. A., Fuortes, M. G. & O'Bryan, P. M. Lateral interaction between vertebrate photoreceptors. Vision Res. 11, 1195–1196 (1971).
Miller, R. F. & Dacheux, R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. I. Intracellular analysis of chloride-sensitive electrogenic properties of receptors, horizontal cells, bipolar cells, and amacrine cells. J. Gen. Physiol 67, 639–659 (1976).
Schwartz, E. A. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science 238, 350–355 (1987).
Byzov, A. L. & Shura-Bura, T. M. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 26, 33–44 (1986).
Kamermans, M. et al. Hemichannel-mediated inhibition in the outer retina. Science 292, 1178–1180 (2001). An imaginative study that provides data supporting a model in which active connexons are the basis for communication of centre-surround antagonism between horizontal cells and cone photoreceptors in the vertebrate retina.
Janssen-Bienhold, U. et al. Identification and localization of connexin26 within the photoreceptor–horizontal cell synaptic complex. Vis. Neurosci. 18, 169–178 (2001).
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Naus, C. C. G., Bond, S. L., Bechberger, J. & Rushlow, W. Identification of genes differentially expressed in C6 glioma cells transfected with connexin43. Brain Res. Brain Res. Rev. 32, 259–266 (2000).
Dahl, G., Nonner, W. & Werner, R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys. J. 67, 1816–1822 (1994).
Kwak, B. R. & Jongsma, H. J. Selective inhibition of gap junction channel activity by synthetic peptides. J. Physiol. (Lond.) 516, 679–685 (1999).
Boitano, S. & Evans, W. H. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L623–L630 (2000).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
Beyer, E. C., Paul, D. L. & Goodenough, D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J. Cell Biol. 105, 2621–2629 (1987).
Kumar, N. M. & Gilula, N. B. Molecular biology and genetics of gap junction channels. Semin. Cell Biol. 3, 3–16 (1992).
Revel, J. -P. & Karnovsky, M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 33, C7–C12 (1967).
Unger, V. M., Kumar, N. M., Gilula, N. B. & Yeager, M. Three-dimensional structure of a recombinant gap junction membrane channel. Science 283, 1176–1180 (1999).
Cho, W. K., Stern, S. & Biggers, J. D. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J. Exp. Zool. 187, 383–386 (1974).
Lawrence, T. S., Beers, W. H. & Gilula, N. B. Transmission of hormonal stimulation by cell-to-cell communication. Nature 272, 501–506 (1978).
Goldberg, G. S. et al. Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp. Cell Res. 239, 82–92 (1998).
Kleopa, K. A., Yum, S. W. & Scherer, S. S. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J. Neurosci. Res. 68, 522–534 (2002).
Balice-Gordon, R. J., Bone, L. J. & Scherer, S. S. Functional gap junctions in the Schwann cell myelin sheath. J. Cell Biol. 142, 1095–1104 (1998).
Vardi, N., Morigiwa, K., Wang, T. L., Shi, Y. J. & Sterling, P. Neurochemistry of the mammalian cone 'synaptic complex'. Vision Res. 38, 1359–1369 (1998).
Acknowledgements
Work in the authors' laboratories is supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Glossary
- METAZOA
-
A subkingdom of animals that includes all multicellular organisms with differentiated tissues.
- HEMICHORDATES
-
A phylum of bilaterally symmetric marine animals with gill slits to the pharynx.
- ACTION POTENTIAL
-
A self-propagating opening of voltage-gated channels along the length of a cell.
- GRANULOSA CELL
-
A cell type found in mammalian ovarian follicles that surrounds the developing oocyte.
- NON-SYNDROMIC DEAFNESS
-
Deafness that is unaccompanied by other clinical manifestations.
- SURFACE-LABELLING
-
The chemical modification of cell-surface molecules using reagents that are membrane impermeant.
- SUCROSE GRADIENT FRACTIONATION
-
The biochemical separation of subcellular components on the basis of their buoyant density.
- CROSSLINKING
-
The use of bifunctional reactive compounds to covalently couple molecules that are in close proximity.
- TELEOST HORIZONTAL CELL
-
A cell type that is found in fish retinas.
- SCHWANN CELL
-
A glial cell in the peripheral nervous system that is responsible for the myelination of axons.
- PARANODAL MEMBRANE
-
A plasma membrane that surrounds non-compact areas of Schwann cells that are found immediately adjacent to nodes of Ranvier.
- INCISURE OF SCHMIDT-LANTERMAN
-
A spiralling region of non-compact myelin that joins the periaxonal with the perinuclear cytoplasm in myelinating glia.
- NOVIKOFF HEPATOMA CELLS
-
A cell line derived from a liver tumour that is used in the study of gap-junctional intercellular communication.
- ASTROCYTES
-
One of several classes of glia (non-neuronal support cells) that are found in the central nervous system.
- VENTRICULAR MYOCYTE
-
A cell that is found in the ventricles of the heart.
- N2A NEUROBLASTOMA CELLS
-
A cell line derived from a brain tumour that has very low endogenous connexin expression, and is useful for studying the introduction of exogenous connexins.
- OLEAMIDE
-
(9(Z)-octadecenamide). A sleep-inducing lipid that was first isolated from the cerebrospinal fluid of sleep-deprived cats. It rapidly and reversibly closes gap-junctional channels.
- 18 α-GLYCYRRHETINIC ACID
-
A lipophilic saponin isolated from liquorice roots that blocks gap-junction channels and connexons.
- C6 GLIOMA CELLS
-
A cell line derived from a glioma that is used to study intercellular communication and Ca2+ waves.
- OSTEOCYTE
-
A differentiated bone cell that is trapped in lamellar bone. Osteocytes are interconnected by gap junctions by processes that extend through long canaliculi.
- P2 PURINERGIC RECEPTOR
-
A cell-surface ATP receptor.
- LIPOSOME
-
A unilamellar vesicle.
- OSTEOBLAST
-
A bone cell that synthesizes and secretes osteoid, which is the extracellular matrix of bone. Osteoblasts differentiate into osteocytes.
- OSTEOGENESIS
-
The process of bone formation.
- OSTEOCLAST
-
A mesenchymal cell that can differentiate into a bone-degrading cell.
- PEDICLE
-
The ending of a cone cell that interacts with other neurons in the retina.
Rights and permissions
About this article
Cite this article
Goodenough, D., Paul, D. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4, 285–295 (2003). https://doi.org/10.1038/nrm1072
Issue Date:
DOI: https://doi.org/10.1038/nrm1072
This article is cited by
-
Robo4 inhibits gamma radiation-induced permeability of a murine microvascular endothelial cell by regulating the junctions
Cellular & Molecular Biology Letters (2023)
-
The circadian regulation of extracellular ATP
Purinergic Signalling (2023)
-
Mechanical regulation of bone remodeling
Bone Research (2022)
-
Mechanotransduction via the coordinated actions of integrins, PI3K signaling and Connexin hemichannels
Bone Research (2021)
-
Connexin Gap Junctions and Hemichannels Link Oxidative Stress to Skeletal Physiology and Pathology
Current Osteoporosis Reports (2021)