Key Points
-
Feedback signalling loops have an essential role in the initiation, amplification, processing and termination of intracellular signals in lymphocytes.
-
Feedback loops cannot be easily identified by classical genetic methods and require special techniques for their detection.
-
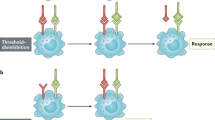
Like a switch with multiple settings, signalling proteins can change their conformation and activity; a process that is regulated by input and feedback signals.
-
Most signalling proteins are allosteric, multidomain proteins consisting of regulatory and functional domains. The regulatory domains have a dual function. On the one hand, they inhibit the signalling activity by autoinhibition. On the other hand, they function as targeting domains that recruit active signalling molecules to the appropriate location in the cell.
-
The simplest form of a positive feedback is a direct enzyme–product feedback loop. One characteristic of a positive feedback is that it becomes independent of the input signal and therefore it has to be tightly regulated both temporally and spatially.
-
The duration of an amplified signal is restricted by negative-feedback loops that serve as important built-in brakes.
-
A double-negative feedback loop is characterized by the inhibition of a signal inhibitor. Such loops are found in bi-stable signalling systems that are characterized by an all-or-nothing output signal.
Abstract
The development, survival and activation of lymphocytes is controlled by a multitude of extracellular signals in the form of soluble or membrane-bound ligands. Binding of these ligands to receptors on the lymphocyte surface is translated into intracellular signals that are processed in various ways inside the cell and determine its fate. The processing of an incoming signal involves amplification, diversification and termination. Feedback signalling loops have an essential role in the control of these processes, yet our knowledge about these regulatory loops is limited. However, several new feedback regulatory circuits have been recently discovered in lymphocytes and it is probable that more of these circuits will be found in the near future. Here, we give an overview of the present knowledge and working principles of such feedback loops.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kurosaki, T. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17, 555–592 (1999).
Mak, T. W., Penninger, J. M. & Ohashi, P. S. Knockout mice: a paradigm shift in modern immunology. Nature Rev. Immunol. 1, 11–19 (2001).
Marshall, C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Adachi, S. & Iwata, M. Duration of calcineurin and Erk signals regulates CD4/CD8 lineage commitment of thymocytes. Cell. Immunol. 215, 45–53 (2002).
Sharp, L. L., Schwarz, D. A., Bott, C. M., Marshall, C. J. & Hedrick, S. M. The influence of the MAPK pathway on T cell lineage commitment. Immunity 7, 609–618 (1997).
Sugawara, T., Moriguchi, T., Nishida, E. & Takahama, Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity 9, 565–574 (1998).
Xu, R., Seger, R. & Pecht, I. Cutting edge: extracellular signal-regulated kinase activates Syk: a new potential feedback regulation of Fcε receptor signaling. J. Immunol. 163, 1110–1114 (1999).
Brummer, T., Naegele, H., Reth, M. & Misawa, Y. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene 22, 8823–8834 (2003).
Rolli, V. et al. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 10, 1057–1069 (2002). By reconstructing B-cell receptor (BCR)-signalling pathways in Drosophila S2 cells, the authors provide insights into the mechanisms of immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation and identify a positive-feedback loop involved in SYK activation.
Saito, K. et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity 19, 669–678 (2003). The authors identify Bruton's tyrosine kinase (BTK) as a stimulator of a positive-feedback loop that not only ensures substrate supply for the BTK activator phosphatidylinositol 3-kinase (PI3K), but also helps to sustain the activity of BTK effectors such as phospholipase C-γ (PLC-γ).
Lynch, D. K. & Daly, R. J. PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2. EMBO J. 21, 72–82 (2002).
Lax, I. et al. The docking protein FRS2α controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 10, 709–719 (2002).
Reth, M. & Wienands, J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15, 453–479 (1997).
Ferrell, J. E., Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002).
Pawson, T. & Scott, J. D. Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080 (1997).
Jordan, M. S., Singer, A. L. & Koretzky, G. A. Adaptors as central mediators of signal transduction in immune cells. Nature Immunol. 4, 110–116 (2003).
Schlessinger, J. Signal transduction. Autoinhibition control. Science 300, 750–752 (2003).
Harrison, S. C. Variation on an Src-like theme. Cell 112, 737–740 (2003).
Hof, P., Pluskey, S., Dhe-Paganon, S., Eck, M. J. & Shoelson, S. E. Crystal structure of the tyrosine phosphatase SHP-2. Cell 92, 441–450 (1998).
Stofega, M. R., Argetsinger, L. S., Wang, H., Ullrich, A. & Carter-Su, C. Negative regulation of growth hormone receptor/JAK2 signaling by signal regulatory protein α. J. Biol. Chem. 275, 28222–28229 (2000).
Neel, B. G., Gu, H. & Pao, L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284–293 (2003).
Blasioli, J., Paust, S. & Thomas, M. L. Definition of the sites of interaction between the protein tyrosine phosphatase SHP-1 and CD22. J. Biol. Chem. 274, 2303–2307 (1999).
Meng, T. C., Fukada, T. & Tonks, N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 (2002).
Takata, M. et al. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13, 1341–1349 (1994).
Cornall, R. J., Cheng, A. M., Pawson, T. & Goodnow, C. C. Role of Syk in B-cell development and antigen-receptor signaling. Proc. Natl Acad. Sci. USA 97, 1713–1718 (2000).
Kurosaki, T. et al. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 182, 1815–1823 (1995).
Keshvara, L. M., Isaacson, C., Harrison, M. L. & Geahlen, R. L. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J. Biol. Chem. 272, 10377–10381 (1997).
Furlong, M. T. et al. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim. Biophys. Acta 1355, 177–190 (1997).
Hong, J. J., Yankee, T. M., Harrison, M. L. & Geahlen, R. L. Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase. J. Biol. Chem. 277, 31703–31714 (2002).
Yankee, T. M., Keshvara, L. M., Sawasdikosol, S., Harrison, M. L. & Geahlen, R. L. Inhibition of signaling through the B cell antigen receptor by the protooncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J. Immunol. 163, 5827–5835 (1999).
Flaswinkel, H. & Reth, M. Dual role of the tyrosine activation motif of the Ig-α protein during signal transduction via the B cell antigen receptor. EMBO J. 13, 83–89 (1994).
Saijo, K. et al. Essential role of Src-family protein tyrosine kinases in NF-κB activation during B cell development. Nature Immunol. 4, 274–279 (2003).
Kraus, M. et al. Interference with immunoglobulin (Ig)α immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igβ cytoplasmic tail. J. Exp. Med. 194, 455–469 (2001).
Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J. & Der, C. J. Increasing complexity of Ras signaling. Oncogene 17, 1395–1413 (1998).
Hancock, J. F. Ras proteins: different signals from different locations. Nature Rev. Mol. Cell Biol. 4, 373–384 (2003).
Hamad, N. M. et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 16, 2045–2057 (2002).
Shaw, A. C., Swat, W., Ferrini, R., Davidson, L. & Alt, F. W. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J. Exp. Med. 189, 123–129 (1999).
Gold, M. R. To make antibodies or not: signaling by the B-cell antigen receptor. Trends Pharmacol. Sci. 23, 316–324 (2002).
Alberola-Ila, J. & Hernandez-Hoyos, G. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191, 79–96 (2003).
Dower, N. A. et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature Immunol. 1, 317–321 (2000).
Priatel, J. J., Teh, S. J., Dower, N. A., Stone, J. C. & Teh, H. S. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17, 617–627 (2002).
Oh-Hora, M., Johmura, S., Hashimoto, A., Hikida, M. & Kurosaki, T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198, 1841–1851 (2003).
Margarit, S. M. et al. Structural evidence for feedback activation by Ras. GTP of the Ras-specific nucleotide exchange factor SOS. Cell 112, 685–695 (2003). Crystallographic analyses showing a ternary son-of-sevenless (SOS)–RAS complex with one RAS molecule bound to the catalytic module of SOS and one RAS-GTP molecule bound to a regulatory site distal to the active site of SOS. This indicates that RAS-GTP stabilizes the active site of SOS allosterically and mediates a positive-feedback mechanism for the spatial and temporal regulation of RAS.
Fruman, D. A., Satterthwaite, A. B. & Witte, O. N. Xid-like phenotypes: a B cell signalosome takes shape. Immunity 13, 1–3 (2000).
Schwartzberg, P. L. Amplifying Btk's signal. Immunity 19, 634–636 (2003).
Healy, J. I. & Goodnow, C. C. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16, 645–670 (1998).
Galm, O., Yoshikawa, H., Esteller, M., Osieka, R. & Herman, J. G. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 101, 2784–2788 (2003).
Hibbs, M. L. et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J. Exp. Med. 196, 1593–1604 (2002).
Cornall, R. J. et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity 8, 497–508 (1998).
O'Keefe, T. L., Williams, G. T., Davies, S. L. & Neuberger, M. S. Hyperresponsive B cells in CD22-deficient mice. Science 274, 798–801 (1996).
Nitschke, L., Carsetti, R., Ocker, B., Kohler, G. & Lamers, M. C. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7, 133–143 (1997). References 48 to 51 provide detailed genetic and biochemical analyses of the LYN–CD22–SHP1 negative-feedback loop in B cells.
Kang, S. W. et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20, 5692–5702 (2001). This work provides a molecular mechanism by which protein kinase C-β (PKC-β) downregulates BCR-induced BTK activity through serine phosphorylation of BTK as part of a negative-feedback loop.
Cochet, C., Gill, G. N., Meisenhelder, J., Cooper, J. A. & Hunter, T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J. Biol. Chem. 259, 2553–2558 (1984).
Muller, R., Wienands, J. & Reth, M. The serine and threonine residues in the Ig-α cytoplasmic tail negatively regulate immunoreceptor tyrosine-based activation motif-mediated signal transduction. Proc. Natl Acad. Sci. USA 97, 8451–8454 (2000).
Losch, F. O. et al. Activation of T cells via tumor antigen specific chimeric receptors: the role of the intracellular signaling domain. Int. J. Cancer 103, 399–407 (2003).
Allouche, M. Basic fibroblast growth factor and hematopoiesis. Leukemia 9, 937–42 (1995).
de Haan, G. et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev. Cell 4, 241–251 (2003).
Kronfeld, I., Kazimirsky, G., Gelfand, E. W. & Brodie, C. NGF rescues human B lymphocytes from anti-IgM induced apoptosis by activation of PKCζ. Eur. J. Immunol. 32, 136–143 (2002).
Gu, H. & Neel, B. G. The 'Gab' in signal transduction. Trends Cell Biol. 13, 122–130 (2003).
Herrera, R. & Sebolt-Leopold, J. S. Unraveling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends Mol. Med. 8, S27–S31 (2002).
Iritani, B. M., Alberola-Ila, J., Forbush, K. A. & Perimutter, R. M. Distinct signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity 10, 713–722 (1999).
Rui, L., Vinuesa, C. G., Blasioli, J. & Goodnow, C. C. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nature Immunol. 4, 594–600 (2003).
Fleming, H. E. & Paige, C. J. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity 15, 521–531 (2001).
Flemming, A., Brummer, T., Reth, M. & Jumaa, H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nature Immunol. 4, 38–43 (2003).
Gardner, A. M., Vaillancourt, R. R., Lange-Carter, C. A. & Johnson, G. L. MEK-1 phosphorylation by MEK kinase, Raf, and mitogen-activated protein kinase: analysis of phosphopeptides and regulation of activity. Mol. Biol. Cell 5, 193–201 (1994).
Mansour, S. J. et al. Mitogen-activated protein (MAP) kinase phosphorylation of MAP kinase kinase: determination of phosphorylation sites by mass spectrometry and site-directed mutagenesis. J. Biochem. (Tokyo) 116, 304–314 (1994).
Clemens, J. C. et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl Acad. Sci. USA 97, 6499–6503 (2000).
Roy, F., Laberge, G., Douziech, M., Ferland-McCollough, D. & Therrien, M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16, 427–438 (2002).
Alessi, D. R., Cuenda, A., Cohen, P., Dudley, D. T. & Saltiel, A. R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270, 27489–27494 (1995).
Wartmann, M., Hofer, P., Turowski, P., Saltiel, A. R. & Hynes, N. E. Negative modulation of membrane localization of the Raf-1 protein kinase by hyperphosphorylation. J. Biol. Chem. 272, 3915–3923 (1997).
Brummer, T., Shaw, P. E., Reth, M. & Misawa, Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 21, 5611–5622 (2002).
Ueki, K. et al. Feedback regulation of mitogen-activated protein kinase kinase kinase activity of c-Raf-1 by insulin and phorbol ester stimulation. J. Biol. Chem. 269, 15756–15761 (1994).
Waters, S. B. et al. Desensitization of Ras activation by a feedback disassociation of the SOS–Grb2 complex. J. Biol. Chem. 270, 20883–20886 (1995).
Corbalan-Garcia, S., Yang, S. S., Degenhardt, K. R. & Bar-Sagi, D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol. Cell. Biol. 16, 5674–5682 (1996).
Douville, E. & Downward, J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene 15, 373–383 (1997).
Harmer, S. L. & DeFranco, A. L. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in B lymphocytes. Mol. Cell. Biol. 17, 4087–4095 (1997).
D'Angelo, G. et al. 16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells. Mol. Endocrinol. 13, 692–704 (1999).
Waters, S. B., Yamauchi, K. & Pessin, J. E. Insulin-stimulated disassociation of the SOS–Grb2 complex. Mol. Cell. Biol. 15, 2791–2799 (1995).
Buday, L., Warne, P. H. & Downward, J. Downregulation of the Ras activation pathway by MAP kinase phosphorylation of Sos. Oncogene 11, 1327–1331 (1995).
Zhang, W., Sloan-Lancaster, J., Kitchen, J., Trible, R. P. & Samelson, L. E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92, 83–92 (1998).
Langlois, W. J., Sasaoka, T., Saltiel, A. R. & Olefsky, J. M. Negative feedback regulation and desensitization of insulin- and epidermal growth factor-stimulated p21ras activation. J. Biol. Chem. 270, 25320–25323 (1995).
Alexander, W. S. Suppressors of cytokine signalling (SOCS) in the immune system. Nature Rev. Immunol. 2, 410–416 (2002).
Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science 298, 1241–1245 (2002).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nature Immunol. 3, 1129–1134 (2002).
Watts, J. D., Sanghera, J. S., Pelech, S. L. & Aebersold, R. Phosphorylation of serine 59 of p56lck in activated T cells. J. Biol. Chem. 268, 23275–23282 (1993).
Joung, I. et al. Modification of Ser59 in the unique N-terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc. Natl Acad. Sci. USA 92, 5778–5782 (1995).
Winkler, D. G. et al. Phosphorylation of Ser-42 and Ser-59 in the N-terminal region of the tyrosine kinase p56lck. Proc. Natl Acad. Sci. USA 90, 5176–5180 (1993).
Stefanova, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nature Immunol. 4, 248–254 (2003). This paper describes how T-cell receptor (TCR)-induced LCK activity is balanced by positive- and negative-feedback loops and provides a physiological relevance for the extracellular signal-regulated kinase (ERK)-mediated feedback phosphorylation of LCK that was discovered a decade earlier.
Schebesta, M., Heavey, B. & Busslinger, M. Transcriptional control of B-cell development. Curr. Opin. Immunol. 14, 216–223 (2002).
Shaffer, A. L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Lin, K. I., Tunyaplin, C. & Calame, K. Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol. Rev. 194, 19–28 (2003).
Moriyama, M., Yamochi, T., Semba, K., Akiyama, T. & Mori, S. BCL-6 is phosphorylated at multiple sites in its serine- and proline-clustered region by mitogen-activated protein kinase (MAPK) in vivo. Oncogene 14, 2465–2474 (1997).
Niu, H., Ye, B. H. & Dalla-Favera, R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 12, 1953–1961 (1998).
Calame, K. L. Plasma cells: finding new light at the end of B cell development. Nature Immunol. 2, 1103–1108 (2001).
Rao, N., Dodge, I. & Band, H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J. Leukoc. Biol. 71, 753–763 (2002).
Jang, I. K. & Gu, H. Negative regulation of TCR signaling and T-cell activation by selective protein degradation. Curr. Opin. Immunol. 15, 315–320 (2003).
Thien, C. B. & Langdon, W. Y. Cbl: many adaptations to regulate protein tyrosine kinases. Nature Rev. Mol. Cell Biol. 2, 294–307 (2001).
Watts, J. D. et al. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J. Biol. Chem. 269, 29520–29529 (1994).
Murphy, M. A. et al. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell Biol. 18, 4872–4882 (1998).
Naramura, M., Kole, H. K., Hu, R. J. & Gu, H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl Acad. Sci. USA 95, 15547–15552 (1998).
Tognon, C. E. et al. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol. Cell. Biol. 18, 6995–7008 (1998).
Su, Y. W. & Jumaa, H. LAT links the pre-BCR to calcium signaling. Immunity 19, 295–305 (2003).
Niiro, H., Maeda, A., Kurosaki, T. & Clark, E. A. The B lymphocyte adaptor molecule of 32 kD (Bam32) regulates B cell antigen receptor signaling and cell survival. J. Exp. Med. 195, 143–149 (2002).
Han, A., Saijo, K., Mecklenbrauker, I., Tarakhovsky, A. & Nussenzweig, M. C. Bam32 links the B cell receptor to ERK and JNK and mediates B cell proliferation but not survival. Immunity 19, 621–632 (2003).
Reynolds, L. F. et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos and RasGRP1. J. Biol. Chem. (in the press)
Adachi, T. et al. SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates. J. Biol. Chem. 276, 26648–26655 (2001).
Mizuno, K. et al. Src homology region 2 (SH2) domain-containing phosphatase-1 dephosphorylates B cell linker protein/SH2 domain leukocyte protein of 65 kDa and selectively regulates c-Jun NH2-terminal kinase activation in B cells. J. Immunol. 165, 1344–1351 (2000).
Pani, G., Kozlowski, M., Cambier, J. C., Mills, G. B. & Siminovitch, K. A. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J. Exp. Med. 181, 2077–2084 (1995).
Cyster, J. G. & Goodnow, C. C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity 2, 13–24 (1995).
Doody, G. M. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995).
Acknowledgements
We thank M. Huber and P. Nielson for critical reading of this review. This work is supported by the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ALLOSTERIC PROTEINS
-
Proteins that contain two or more topologically distinct binding sites, which interact functionally with each other; for example, binding of a ligand, often a product or downstream effector of the protein, to one site affects the activity or accessibility of the other site.
- SRC-HOMOLOGY 2 DOMAIN
-
(SH2 domain). A protein domain commonly found in signal-transduction molecules that interacts specifically with phosphotyrosine-containing peptides.
- SRC-HOMOLOGY 3 DOMAIN
-
(SH3 domain). A protein domain that binds proline-rich peptide sequences with the consensus Pro-Xaa-Xaa-Pro. The binding between SH3 domains and their target sequence is often regulated by serine/threonine phosphorylation of the target protein close to the recognition site.
- PLECKSTRIN-HOMOLOGY DOMAIN
-
(PH domain). A protein domain that binds to the charged headgroups of specific polyphosphoinositides and thereby targets signalling proteins to specific regions of the plasma membrane enriched by these lipids.
- IMMUNORECEPTOR TYROSINE-BASED INHIBITORY MOTIF
-
(ITIM). This motif is found in the cytoplasmic domains of inhibitory receptors. After ligand binding, the ITIM (Val/Ile-Xaa-Tyr-Xaa-Xaa-Leu/Val) becomes tyrosine phosphorylated, which recruits and activates phosphatases.
- IMMUNORECEPTOR TYROSINE-BASED ACTIVATION MOTIF
-
(ITAM). A structural motif containing tyrosine residues, found in the cytoplasmic tails of several activating receptors. The motif contains two tyrosines in the sequence context Tyr-Xaa-Xaa-Leu/Ile. After tyrosine phosphorylation, the motif becomes a binding target for SRC-homology 2-domain-containing proteins.
- UBIQUITYLATION
-
Covalent conjugation of the highly conserved protein ubiquitin to a target protein via an isopeptide bond between its carboxyl terminus and the ε-amino group of a lysine residue of the target protein. Ubiquitylation is often triggered by phosphorylation of the target protein close to the acceptor lysine residue. Ubiquitylation can fix an allosteric protein in a specific conformation or target it for degradation by the proteasome complex.
- JAK/STAT PATHWAY
-
The Janus-family kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is activated by numerous cytokines and growth factors. JAKs associate with cytokine receptors and become activated after ligand binding. Activated JAKs tyrosine phosphorylate the receptor and recruit STATs, which are also phosphorylated by activated JAKs. Phosphorylated STATs then dimerize and translocate to the nucleus, where they control gene transcription.
- SOCS PROTEINS
-
The suppressors of cytokine signalling (SOCS) are a family of intracellular proteins that modulate the activity of cytokine receptors and receptor tyrosine kinases. SOCS proteins seem to regulate signal transduction by either direct inhibitory interactions with cytokine receptors and Janus-family kinases or by targeting associated proteins for degradation.
Rights and permissions
About this article
Cite this article
Reth, M., Brummer, T. Feedback regulation of lymphocyte signalling. Nat Rev Immunol 4, 269–278 (2004). https://doi.org/10.1038/nri1335
Issue Date:
DOI: https://doi.org/10.1038/nri1335
This article is cited by
-
Toll-like receptor signalling in B cells during systemic lupus erythematosus
Nature Reviews Rheumatology (2021)
-
Genetics ignite focus on microglial inflammation in Alzheimer’s disease
Molecular Neurodegeneration (2015)
-
Multi-Stability and Multi-Instability Phenomena in a Mathematical Model of Tumor-Immune-Virus Interactions
Bulletin of Mathematical Biology (2011)
-
Tyrosine phosphatase SHP-1 is expressed higher in multisystem than in single-system Langerhans cell histiocytosis by immunohistochemistry
Virchows Archiv (2011)
-
Pairing computation with experimentation: a powerful coupling for understanding T cell signalling
Nature Reviews Immunology (2010)