Key Points
-
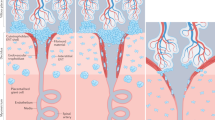
The main population of lymphoid cells in the mucosa of the uterus during early pregnancy are CD56bright natural killer (NK) cells. Their number decreases from mid-gestation onwards and they are absent at term.
-
Uterine NK (uNK) cells are influenced by sex hormones, particularly progesterone. There is recent evidence that endometrial-derived interleukin-15 and prolactin also affect their proliferation and differentiation.
-
All uNK cells express high levels of the inhibitory CD94/NKG2A receptor, the ligand for which is HLA-E. Killer-cell immunoglobulin-like receptors (KIRs) that are specific for HLA-C are also expressed by a higher proportion of uNK cells than by peripheral-blood NK cells in pregnant women, which indicates that the NK-cell receptor repertoire is skewed towards recognition of HLA-C in the uterus and could be induced by the local environment. Other receptors, such as KIR2DL4 and immunoglobulin-like transcript 2 (ILT2), the ligand for which is thought to be HLA-G, are also expressed by uNK cells.
-
As HLA-C, HLA-E and HLA-G are expressed by the population of extravillous trophoblast (EVT) cells that invades the uterus, it has been proposed that interaction between these trophoblast MHC class I molecules and uNK-cell receptors could be important for the control of implantation.
-
HLA-E would be expected to be the overall inhibitory ligand through interaction with CD94/NKG2A. Interestingly, the presence of a peptide that is derived from the leader sequence of HLA-G can influence the binding of HLA-E to its receptor. As HLA-G is expressed only by EVT, this is one way in which trophoblast can specifically influence uNK-cell activity.
-
HLA-C is the only one of the three trophoblast MHC class I molecules that is polymorphic. As all women express KIRs for both groups of HLA-C alleles, the recognition of paternal non-self HLA-C should occur during pregnancy. It is possible that some combinations of paternal HLA-C and maternal KIRs are sub-optimal for implantation, leading to diseases such as pre-eclampsia, for which partner specificity, and correlation with first pregnancy and oocyte donation are characteristic features of an immunological pathogenesis.
-
Although the interaction between trophoblast class I molecules and uNK cells is likely to provide the pivotal control for successful implantation, it is unclear how it is mediated. Uterine NK cells might have a dual role to play in reproduction, by monitoring mucosal integrity throughout the menstrual cycle and controlling trophoblast invasion during pregnancy.
-
The extraordinary species diversity in the anatomy of placentation has made direct comparison between humans and animals difficult. Nevertheless, much can be deduced from animal studies. Uterine NK cells from different species might have evolved different mechanisms to support successful reproduction.
-
The fetus and placenta have traditionally been considered to be analogous to an allograft. However, it is the evolutionarily older innate immune system that seems to have a dominant influence on reproduction. Conceptually, this is an important paradigm shift and offers a new insight into the immunology of reproduction.
Abstract
The fetus is considered to be an allograft that, paradoxically, survives pregnancy despite the laws of classical transplantation immunology. There is no direct contact of the mother with the embryo, only with the extraembryonic placenta as it implants in the uterus. No convincing evidence of uterine maternal T-cell recognition of placental trophoblast cells has been found, but instead, there might be maternal allorecognition mediated by uterine natural killer cells that recognize unusual fetal trophoblast MHC ligands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Beer, A. E. & Billingham, R. E. The Immunobiology of Mammalian Reproduction (Prentice–Hall, New Jersey, USA, 1976).
Loke, Y. W. & King, A. Human Implantation: Cell Biology and Immunology (Cambridge University Press, Cambridge, UK, 1995).
King, A., Burrows, T., Verma, S., Hiby, S. & Loke, Y. W. Human uterine lymphocytes. Hum. Reprod. Update 4, 480–485 (1998).References 2 and 3 provide detailed background information on the cellular relationships that occur during placentation.
Trowsdale, J. Genetic and functional relationships between MHC and NK receptor genes. Immunity 15, 363–374 (2001).A concise and comprehensive review of MHC and NK-cell receptor genes.
Pijnenborg, R., Bland, J. M., Robertson, W. B. & Brosens, I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4, 397–414 (1983).
Pijnenborg, R. Trophoblast invasion. Reprod. Med. Rev. 3, 53–73 (1994).A detailed review of how placental cells invade maternal arteries.
Brosens, I. A. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin. Obstet. Gynaecol. 4, 573–593 (1977).
Khong, T. Y., De Wolf, F., Robertson, W. B. & Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 93, 1049–1059 (1986).
King, A. Uterine leukocytes and decidualization. Hum. Reprod. Update 6, 28–36 (2000).
Gubbay, O., Critchley, H. O. D., King, A., Bowen, J. M. & Jabbour, H. N. Prolactin induces ERK phosphorylation in epithelial and CD56+ natural killer cells of the human endometrium. J. Clin. Endocrinol. Metab. 87, 2329–2335 (2002).
Dunn, C. L., Critchley, H. O. D. & Kelly, R. W. IL-15 regulation in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 87, 1898–1901 (2002).
Spornitz, U. M. The functional morphology of the human endometrium and decidua. Adv. Anat. Embryol. Cell Biol. 124, 1–99 (1992).
Li, X. F. et al. Angiogenic growth factor mRNAs in uterine natural killer cells. J. Clin. Endocrinol. Metab. 86, 1823–1834 (2001).
Wheeler, J. E., Gersell, D. J. & Kraus, F. T. in Blaustein's Pathology of the Female Genital Tract (ed. Kurman, R. J.) 630–633 & 1111–1113 (Springer, New York, 2002).
Ellis, S. A., Palmer, M. S. & McMichael, A. J. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J. Immunol. 144, 731–735 (1990).
Kovats, S. et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248, 220–223 (1990).
King, A., Boocock, C., Sharkey, A., Gardner, L. & Loke, Y. W. Evidence for the expression of HLA-C class I mRNA and protein by human first trimester trophoblast. J. Immunol. 156, 2068–2076 (1996).
King, A. et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta 21, 376–387 (2000).
Taruscio, D. & Mantovani, A. Human endogenous retroviral sequences: possible roles in reproductive physiopathology. Biol. Reprod. 59, 713–724 (1998).
Harris, J. R. Placental endogenous retrovirus (ERV): structural, functional and evolutionary significance. BioEssays 20, 307–316 (1998).
Loke, Y. W. in Immunology of Pregnancy and its Disorders (ed. Stern, C.) 61–89 (Kluwer Academic Publishers, Dordrecht, The Netherlands, 1988).
Brummer, J. et al. Interaction of the cell adhesion molecule CEACAM1 with integrin-β3. Am. J. Pathol. 159, 537–546 (2001).
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Lee, N. et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl Acad. Sci. USA 95, 5199–5204 (1998).
Boyington, J. C., Brooks, A. G. & Sun, P. D. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol. Rev. 181, 52–65 (2001).
Long, E. O. et al. Inhibition of natural killer cell activation signals by killer cell immunoglobulin-like receptors (CD158). Immunol. Rev. 181, 223–233 (2001).
Vilches, C. & Parham, P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 20, 217–251 (2002).A comprehensive review of KIR genes.
King, A. et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30, 1623–1631 (2000).
Haedicke, W. et al. Expression of CD94/NKG2A and killer immunoglobulin-like receptors in NK cells and a subset of extranodal cytotoxic T-cell lymphomas. Blood 95, 3628–3630 (2000).
Llano, M. et al. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 28, 2854–2863 (1998).
Vales-Gomez, M., Reburn, H. T., Erskine, R. A., Lopez-Botet, M. & Strominger, J. L. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 18, 4250–4260 (1999).
McMaster, M. et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154, 3771–3778 (1995).
Loke, Y. W. et al. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens 50, 135–146 (1997).
Suarez, M. B. et al. A new HLA-G allele (HLA-G*0105N) and its distribution in the Spanish population. Immunogenetics 45, 464–465 (1997).
Arnaiz-Villena, A. et al. The evolution of the MHC-G gene does not support a functional role for the complete protein. Immunol. Rev. 183, 65–75 (2001).
Uhrberg, M. et al. Human diversity in killer cell inhibitory genes. Immunity 7, 753–763 (1997).
Rajalingam, R., Gardiner, C. M., Canavez, F., Vilches, C. & Parham, P. Identification of seventeen novel KIR variants: fourteen of them from non-Caucasian donors. Tissue Antigens 57, 22–31 (2001).
Wilson, M. et al. Plasticity in the organisation and sequences of human KIT/ILKT families. Proc. Natl Acad. Sci. USA 97, 4778–4783 (2000).
Rajalingam, R. et al. Short KIR haplotypes in pygmy chimpanzee (bonobo) resemble the conserved framework of diverse human KIR haplotypes. J. Exp. Med. 193, 135–146 (2001).
Khakoo, S. I. et al. Rapid evolution of NK-cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity 12, 687–698 (2000).
Hiby, S. E., King, A., Sharkey, A. M. & Loke, Y. W. Human uterine cells have a similar repertoire of killer inhibitory and activatory receptors to those found in blood as demonstrated by RT-PCR and sequencing. Mol. Immunol. 34, 419–430 (1997).
Verma, S., King, A. & Loke, Y. W. Expression of killer-cell inhibitory receptors (KIR) on human uterine NK cells. Eur. J. Immunol. 27, 979–983 (1997).
Valiante, N. M. et al. Functionally and structurally distinct NK-cell receptor repertoires in the peripheral blood of two human donors. Immunity 7, 739–751 (1997).
Jacobs, R. et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 31, 3121–3126 (2001).
Lanier, L. L. Natural killer cells fertile with receptors for HLA-G? Proc. Natl Acad. Sci. USA 96, 5343–5345 (1999).
Rajagopalan, S. & Long, E. O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100 (1999).
Ponte, M. et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, and HLA-G1-specific receptor. Proc. Natl Acad. Sci. USA 96, 5674–5679 (1999).
Rajagopalan, S., Fu, J. & Long, E. O. Induction of IFN-γ production but not cytotoxicity by the killer-cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J. Immunol. 167, 1877–1881 (2001).References 46–48 describe the expression and function of KIR2DL4.
Allan, D. S. J. et al. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA-G) bind to peripheral-blood myelomonocytic cells. J. Exp. Med. 189, 1–7 (1999).
Navarro, F. et al. NK-cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur. J. Immunol. 29, 277–283 (1999).
Chang, C. C. et al. Tolerization of dendritic cells by Ts cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature Immunol. 3, 237–243 (2002).
McMaster, M. et al. HLA-G isoforms produced by placental cytotrophoblasts and found in amniotic fluid are due to unusual glycosylation. J. Immunol. 160, 5922–5928.
King, A. & Loke, Y. W. On the nature and function of human uterine granular lymphocytes. Immunol. Today 12, 432–435 (1991).
Avril, T. et al. Trophoblast cell line resistance to NK lysis mainly involves an HLA class-I-independent mechanism. J. Immunol. 162, 5902–5909 (1999).
Norwitz, E. R., Schust, D. J. & Fisher, S. J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408 (2001).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
King, A., Thomas, L. & Bischoff, P. Cell-culture models of trophoblast. II. Trophoblast cell lines — a workshop report. Trophoblast Res. 14, S113–S119 (2000).
Sharkey, A. M. et al. Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biol. Reprod. 60, 355–364 (1999).
Steven, D. H. Comparative Placentation, Essays in Structure and Function (Academic Press, London, 1975).
Liu, C.-C. & Young, J. D.-E. Uterine natural killer cells in the pregnant uterus. Adv. Immunol. 79, 297–329 (2001).
Rossant, J. & Cross, J. C. Placental development: lessons from mouse mutants. Nature Rev. Genet. 2, 538–548 (2001).
Georgiades, P., Ferguson-Smith, A. C. & Burton, G. J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002).
Chantakru, S. et al. Contributions from self-renewal and trafficking to the uterine NK-cell population of early pregnancy. J. Immunol. 168, 22–28 (2002).This article describes homing experiments of uterine NK cells in mice.
Kruse, A., Martens, N., Fernekorn, U., Hallmann, R. & Butcher, E. C. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol. Reprod. 66, 333–345 (2002).
Burrows, T. D., King, A. & Loke, Y. W. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta 15, 21–33 (1994).
Burrows, T. D., King, A. & Loke, Y. W. Expression of adhesion molecules by human decidual large granular lymphocytes. Cell. Immunol. 147, 81–94 (1993).
Guimond, M.-J., Wang, B. & Croy, B. A. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficit in natural killer cell-deficient tgɛ mice. J. Exp. Med. 187, 217–223 (1998).
Greenwood, J. D. et al. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 21, 693–702 (2000).
Ashkar, A. A. & Croy, B. A. Functions of uterine natural killer cells are mediated by interferon-γ production during murine pregnancy. Semin. Immunol. 13, 235–241 (2001).This study describes changes in mice that are deficient in uterine NK cells.
Boyson, J. E., Iwanaga, K. K., Golos, R. G. & Watkins, D. I. Identification of the rhesus monkey HLA-G ortholog. Mamu-G is a pseudogene. J. Immunol. 157, 5428–5437 (1996).
Adams, E. J. & Parham, P. Species-specific evolution of MHC class I genes in the higher primates. Immunol. Rev. 183, 41–64 (2001).
Cooper, M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97, 3146–3151 (2001).
Gieselhart, A. et al. Comparative analysis of the phenotypes of decidual and peripheral blood large granular lymphocytes and T cells during early human pregnancy. Am. J. Reprod. Immunol. 33, 315–322 (1995).
Schalhammer, L., Walcher, W., Wintersteiger, R., Dohr, G. & Sedlmayr, P. Phenotypic comparison of natural killer cells from peripheral blood and early pregnancy decidua. Early Pregnancy 3, 15–22 (1997).
Acknowledgements
I am grateful to Y. W. Loke for comments on the manuscript and to J. Connor for secretarial help. Research in my laboratory is supported by the Wellcome Trust, the National Institutes of Health, the British Heart Foundation and the Isaac Newton Trust.
Author information
Authors and Affiliations
Glossary
- VIVIPARITY
-
The bearing of live young who derive nutrition directly from the mother.
- VILLOUS TROPHOBLAST
-
The trophoblast cells that cover the placental villi, which project into the intervillous space that is perfused with maternal blood.
- PRE-ECLAMPSIA
-
A disease that is specific to pregnancy that is caused by the presence of the placenta. It is characterized by diverse systemic symptoms that are triggered by endothelial dysfunction, which commonly include hypertension and proteinuria. The incidence varies from 3–10% of pregnancies, and this disease is the leading cause of maternal mortality and fetal morbidity and mortality throughout the world.
- CORPUS LUTEUM
-
An endocrine gland that produces both oestrogen and progesterone, which is formed from the wall of the Graafian follicle after ovulation has occurred.
- ONCOFETAL ANTIGENS
-
Antigens that are normally expressed only during early development, but that are re-expressed by malignant cells in adults.
- TETRAMER
-
A soluble reagent that consists of a fluorophore-conjugated core surrounded by four peptide–MHC complexes.
- HAEMOCHORIAL
-
A form of placentation in which trophoblast cells erode the maternal vasculature, which results in the direct contact of maternal blood with trophoblast.
- STAMPER WOODRUFF ASSAY
-
An assay in which the adherence of lymphocytes to tissue-specific endothelial cells is studied using tissue sections.
- VASCULAR ADRESSINS
-
Adhesion molecules that are differentially expressed on endothelial cells in various anatomical locations; they bind to cognate ligands on leukocyte subsets and direct their homing to specific microenvironments.
Rights and permissions
About this article
Cite this article
Moffett-King, A. Natural killer cells and pregnancy. Nat Rev Immunol 2, 656–663 (2002). https://doi.org/10.1038/nri886
Issue Date:
DOI: https://doi.org/10.1038/nri886
This article is cited by
-
Die Physiologie des Implantationsprozesses
Gynäkologische Endokrinologie (2024)
-
Centromeric AA motif in KIR as an optimal surrogate marker for precision definition of alloimmune reproductive failure
Scientific Reports (2024)
-
HLA-E restricted cytomegalovirus UL40 peptide polymorphism may represent a risk factor following congenital infection
BMC Genomics (2022)
-
Premature delivery in the domestic sow in response to in utero delivery of AAV9 to fetal piglets
Gene Therapy (2022)
-
Investigation of interleukin-2-mediated changes in blood pressure, fetal growth restriction, and innate immune activation in normal pregnant rats and in a preclinical rat model of preeclampsia
Biology of Sex Differences (2021)