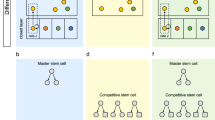
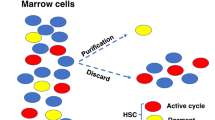
Abstract
Stem cells are endowed with self-renewal and multipotential differentiation capacities. Contrary to the expectation that stem cells would selectively express specific genes, these cells have a highly promiscuous gene-expression pattern. Here, I suggest that the transient stem cell state, termed the 'stem state', may be assumed by any cell and that the search for specific genes expressed by all stem cells, which would characterize the stem cell as a cell type, might be futile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jiang, Y. et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49 (2002).
Phillips, R. L. et al. The genetic program of hematopoietic stem cells. Science 288, 1635–1640 (2000).
Ivanova, N. B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002).
Fortunel, N. O. et al. Comment on '“Stemness”: transcriptional profiling of embryonic and adult stem cells”' and '“A stem cell molecular signature”'. Science 302, 393 (2003).
Evsikov, A. V. & Solter, D. Comment on '“Stemness”: transcriptional profiling of embryonic and adult stem cells' and 'a stem cell molecular signature'. Science 302, 393 (2003).
Matsuzaki, Y., Kinjo, K., Mulligan, R. C. & Okano, H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity 20, 87–93 (2004).
Mazurier, F., Doedens, M., Gan, O. I. & Dick, J. E. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD–SCID mice reveals a new class of human stem cells. Nature Med. 9, 959–963 (2003).
Zipori, D. The renewal and differentiation of hemopoietic stem cells. FASEB J. 6, 2691–2697 (1992).
Back, J., Dierich, A., Bronn, C., Kastner, P. & Chan, S. PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood 103, 3615–3623 (2004).
Muller-Sieburg, C. E., Cho, R. H., Karlsson, L., Huang, J. F. & Sieburg, H. B. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood 103, 4111–4118 (2004).
Younes, S. A. et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198, 1909–1922 (2003).
Trentin, A., Glavieux-Pardanaud, C., Le Douarin, N. M. & Dupin, E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc. Natl Acad. Sci. USA 101, 4495–4500 (2004).
Zipori, D. Regulation of hemopoiesis by cytokines that restrict options for growth and differentiation. Cancer Cells 2, 205–211 (1990).
Hochedlinger, K. & Jaenisch, R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002).
Morrison, S. J., Hemmati, H. D., Wandycz, A. M. & Weissman, I. L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl Acad. Sci. USA 92, 10302–10306 (1995).
Goolsby, J. et al. Hematopoietic progenitors express neural genes. Proc. Natl Acad. Sci. USA 100, 14926–14931 (2003).
Woodbury, D., Reynolds, K. & Black, I. B. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J. Neurosci. Res. 69, 908–917 (2002).
Barda-Saad, M. et al. The mesenchyme expresses T cell receptor mRNAs: relevance to cell growth control. Oncogene 21, 2029–2036 (2002).
Prudhomme, W., Daley, G. Q., Zandstra, P. & Lauffenburger, D. A. Multivariate proteomic analysis of murine embryonic stem cell self–renewal versus differentiation signaling. Proc. Natl Acad. Sci. USA 101, 2900–2905 (2004).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 (2004).
Ding, S. et al. Synthetic small molecules that control stem cell fate. Proc. Natl Acad. Sci. USA 100, 7632–7637 (2003).
Lee, H. Y. et al. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science 303, 1020–1023 (2004).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Langer, J. C., Henckaerts, E., Orenstein, J. & Snoeck, H. W. Quantitative trait analysis reveals transforming growth factor-β2 as a positive regulator of early hematopoietic progenitor and stem cell function. J. Exp. Med. 199, 5–14 (2004).
Gerber, H. P. et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417, 954–958 (2002).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Murdoch, B. et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc. Natl Acad. Sci. USA 100, 3422–3427 (2003).
de Haan, G. et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev. Cell 4, 241–251 (2003).
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003).
Lessard, J. & Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423, 255–260 (2003).
Ueno, H. et al. A stromal cell-derived membrane protein that supports hematopoietic stem cells. Nature Immunol. 4, 457–463 (2003).
Bernstein, E. et al. Dicer is essential for mouse development. Nature Genet. 35, 215–217 (2003).
Harada, H. et al. FGF10 maintains stem cell compartment in developing mouse incisors. Development 129, 1533–1541 (2002).
Kunisada, T. et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development 125, 2915–2923 (1998).
Nishimura, E. K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–8560 (2002).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science 294, 2546–2549 (2001).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Brun, A. C. et al. Hoxb4 deficient mice have normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood 103, 4126–4133 (2004).
Larsson, J. et al. TGF-β signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood 102, 3129–3135 (2003).
Peled, A. et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95, 3289–3296 (2000).
van de Wiel-van Kemenade, E. et al. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the β subunit of VLA. J. Cell Biol. 117, 461–470 (1992).
Song, X. et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353–1364 (2004).
Weigel, D. & Jurgens, G. Stem cells that make stems. Nature 415, 751–754 (2002).
Tanaka, E. M. Regeneration: if they can do it, why can't we? Cell 113, 559–562 (2003).
Prindull, G. & Zipori, D. Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood 103, 2892–2899 (2004).
Zhao, Y., Glesne, D. & Huberman, E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc. Natl Acad. Sci. USA 100, 2426–2431 (2003).
Grafi, G. How cells dedifferentiate: a lesson from plants. Dev. Biol. 268, 1–6 (2004).
Acknowledgements
The author is supported by the Gabrielle Rich Center for Transplantation Biology and by research grants from the Minerva Foundation, Michael Krasny, Daniel and Rhonda Shapiro, Jerrald and Helene Wulff and the Isabelle and Leonard Goldenson Association. The author wishes to thank Ayelet Laron and Vered Morad for their valuable comments on the manuscript. D.Z. is an incumbent of the Joe and Celia Weinstein Professorial Chair at the Weizmann Institute of Science.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez
FURTHER INFORMATION
Glossary
- CYTOKINES
-
A group of proteins that form a dynamic network of intercellular messenger molecules that regulate various aspects of physiology, including the immune response to infection.
- ENDOTHELIAL CELLS
-
Flattened cells that grow in a single layer and line blood vessels.
- G0 PHASE
-
The resting phase of the cell cycle.
- HAEMATOPOIETIC
-
Pertaining to the formation of blood and blood cells.
- MACROPHAGES
-
Phagocytic cells that respond to non-self material (for example, bacteria, protozoa or tumour cells) to release substances that stimulate other cells of the immune system. They are also involved in antigen presentation and are derived from monocytes, which circulate in the blood.
- MESENCHYME
-
Motile, loosely organized unpolarized cells that do not form firm cell–cell contacts and have a proteoglycan-rich extracellular matrix.
- MONOCYTES
-
Large white blood cells involved in the first line of immune defence and in the inflammatory process.
- STROMATA
-
Connective tissue that is made up of cells, such as fibroblasts, and matrix, such as collagen, and forms the supportive organ microenvironment
- T CELLS
-
Lymphoid-derived white blood cells that are responsible for cell-mediated immunity.
Rights and permissions
About this article
Cite this article
Zipori, D. The nature of stem cells: state rather than entity. Nat Rev Genet 5, 873–878 (2004). https://doi.org/10.1038/nrg1475
Issue Date:
DOI: https://doi.org/10.1038/nrg1475
This article is cited by
-
Masking, extrinsicness, and the nature of dispositions: the role of niche signals in muscle stem cells
European Journal for Philosophy of Science (2023)
-
Distinguishing between ante factum and post factum properties of animal cell lines and demonstrating their use in grouping ray-finned fish cell lines into invitromes
In Vitro Cellular & Developmental Biology - Animal (2023)
-
What is the nature of stem cells? A unified dispositional framework
Biology & Philosophy (2023)
-
Applications of mesenchymal stem cell technology in bovine species
Stem Cell Research & Therapy (2019)