Key Points
-
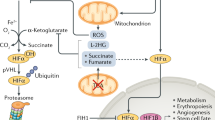
Hypoxia and the cellular hypoxic response have key roles in homeostasis and physiological adaptations, as well as in pathophysiological conditions.
-
The cellular hypoxic response can generate both diversity and specificity in the downstream signalling output, despite a relatively simple core signalling pathway.
-
Hypoxia-inducible factor-α proteins constitute key transcriptional regulators in the cellular hypoxic response, and are subject to various different post-translational modifications.
-
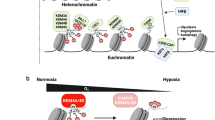
Analysis of the hypoxia transcriptional response has begun to reveal a core hypoxic transcriptional signature in addition to cell type-specific gene activation events.
-
The transcriptional responses to acute and chronic hypoxia are distinct. Likewise, hypoxia-mimicking chemical compounds have a substantially broader transcriptional output than hypoxia.
-
Intersections with other signalling mechanisms, such as Myc and Notch signalling, contribute to modulation of the hypoxic response.
Abstract
The sensing of oxygen levels and maintenance of oxygen homeostasis is crucial for cells. The hypoxic-sensitive regulation of gene expression allows information about the oxygen status to be converted into appropriate cellular responses. Although there is a core transcriptional pathway, the signalling cascade can be modified to allow diversity and specificity in the transcriptional output. In this Review, we discuss recent advances in our understanding of the mechanisms and factors that contribute to the observed diversity and specificity. A deeper knowledge about how hypoxic signalling is tuned will further our understanding of the cellular hypoxic response in normal physiology and how it becomes derailed in disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Smith, T. G., Robbins, P. A. & Ratcliffe, P. J. The human side of hypoxia-inducible factor. Br. J. Haematol. 141, 325–334 (2008).
Weidemann, A. & Johnson, R. S. Biology of HIF-1α. Cell Death Differ. 15, 621–727 (2008).
Simon, M. C., Liu, L., Barnhart, B. C. & Young, R. M. Hypoxia-induced signaling in the cardiovascular system. Annu. Rev. Physiol. 70, 51–71 (2008).
Chan, D. A., Krieg, A. J., Turcotte, S. & Giaccia, A. J. HIF gene expression in cancer therapy. Methods Enzymol. 435, 323–345 (2007).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Denko, N. C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Rev. Cancer 8, 705–713 (2008).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Chen, K. F., Lai, Y. Y., Sun, H. S. & Tsai, S. J. Transcriptional repression of human cad gene by hypoxia inducible factor-1α. Nucleic Acids Res. 33, 5190–5198 (2005).
Mazure, N. M. et al. Repression of α-fetoprotein gene expression under hypoxic conditions in human hepatoma cells: characterization of a negative hypoxia response element that mediates opposite effects of hypoxia inducible factor-1 and c-Myc. Cancer Res. 62, 1158–1165 (2002).
Peyssonnaux, C. et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J. Clin. Invest. 117, 1926–1932 (2007).
Mole, D. R. et al. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 284, 16767–16775 (2009).
Wenger, R. H., Stiehl, D. P. & Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, re12 (2005).
Antonsson, C., Arulampalam, V., Whitelaw, M. L., Pettersson, S. & Poellinger, L. Constitutive function of the basic helix–loop–helix/PAS factor Arnt. Regulation of target promoters via the E box motif. J. Biol. Chem. 270, 13968–13972 (1995).
Sogawa, K. et al. Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc. Natl Acad. Sci. USA 92, 1936–1940 (1995).
Lindebro, M. C., Poellinger, L. & Whitelaw M. L. Protein–protein interaction via PAS domains: role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor–Arnt transcription factor complex. EMBO J. 14, 3528–3539 (1995).
Pongratz, I., Antonsson, C., Whitelaw, M. L. & Poellinger, L. Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. Mol. Cell. Biol. 18, 4079–4088 (1998).
Ruas, J. L., Poellinger, L. & Pereira, T. Role of CBP in regulating HIF-1-mediated activation of transcription. J. Cell Sci. 118, 301–311 (2005).
Carrero, P. et al. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1α. Mol. Cell. Biol. 20, 402–415 (2000).
Kato, H., Tamamizu-Kato, S. & Shibasaki, F. Histone deacetylase 7 associates with hypoxia-inducible factor 1α and increases transcriptional activity. J. Biol. Chem. 279, 41966–41974 (2004).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001).
Kallio, P. J., Pongratz, I., Gradin, K., McGuire, J. & Poellinger, L. Activation of hypoxia-inducible factor 1α: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl Acad. Sci. USA 94, 5667–5672 (1997).
Salceda, S. & Caro, J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272, 22642–22647 (1997).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998).
Kallio, P. J. et al. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1α. EMBO J. 17, 6573–6586 (1998).
Kallio, P. J., Wilson, W. J., O'Brien, S., Makino, Y. & Poellinger, L. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J. Biol. Chem. 274, 6519–6525 (1999).
Stebbins, C. E., Kaelin, W. G. Jr & Pavletich, N. P. Structure of the VHL–ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 (1999).
Tanimoto, K., Makino, Y., Pereira, T. & Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel–Lindau tumor suppressor protein. EMBO J. 19, 4298–4309 (2000).
Ohh, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel–Lindau protein. Nature Cell Biol. 2, 423–427 (2000).
Cockman, M. E. et al. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel–Lindau tumor suppressor protein. J. Biol. Chem. 275, 25733–25741 (2000).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Epstein A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Lando, D. et al. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Hewitson, K. S. et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 277, 26351–26355 (2002).
Carbia-Nagashima, A. et al. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1α during hypoxia. Cell 131, 309–323 (2007).
Cheng, J., Kang, X., Zhang, S. & Yeh, E. T. SUMO-specific protease 1 is essential for stabilization of HIF1α during hypoxia. Cell 131, 584–595 (2007). This paper describes the discovery of a new mechanism for the regulation of VHL–HIFα interaction.
André, H. & Pereira, T. S. Identification of an alternative mechanism of degradation of the hypoxia-inducible factor-1α. J. Biol. Chem. 283, 29375–29384 (2008).
Tojo, M. et al. The aryl hydrocarbon receptor nuclear transporter is modulated by the SUMO-1 conjugation system. J. Biol. Chem. 277, 46576–46585 (2002).
Cardone, L. et al. Circadian clock control by SUMOylation of BMAL1. Science 309, 1390–1394 (2005).
Li, F. et al. Regulation of HIF-1α stability through S-nitrosylation. Mol. Cell 26, 63–74 (2007).
Gradin, K. et al. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol. Cell. Biol. 16, 5221–5231 (1996).
Liu, Y. V. et al. RACK1 competes with HSP90 for binding to HIF-1α and is required for O2-independent and HSP90 inhibitor-induced degradation of HIF-1α. Mol. Cell 25, 207–217 (2007).
Yun, Z., Maecker, H. L., Johnson, R. S. & Giaccia, A. J. Inhibition of PPAR γ 2 expression by the HIF-1-regulated gene DEC1/Stra13. Dev. Cell 2, 331–341 (2002).
Boutin, A. T. et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 132, 223–234 (2008). This paper indicates a role of the skin as a mediator of systemic responses to environmental oxygen.
Botusan, I. R. et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc. Natl Acad. Sci. USA 105, 19426–19431 (2008).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Ryan, H. E., Lo, J. & Johnson, R. S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3015 (1998).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A. & Simon, M. C. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407.
Tian, H., Hammer, R. E., Matsumoto, A. M., Russell, D. W. & McKnight, S. L. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 (1998).
Peng, J., Zhang, L. Y., Drysdale L. & Fong, G. H. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc. Natl Acad. Sci. USA 97, 8386–8391 (2000).
Compernolle, V. et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature Med. 8, 702–710 (2002).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genet. 35, 331–340 (2003).
Morita, M. et al. HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 22, 1134–1146 (2003).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003). Myeloid-specific gene targeting identifies an essential role for HIF1α in regulating inflammatory responses.
Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 453, 807–811 (2008).
Yamashita, T. et al. Abnormal heart development and lung remodeling in mice lacking the hypoxia-inducible factor-related basic helix–loop–helix PAS protein NEPAS. Mol. Cell. Biol. 28, 1285–1297 (2008).
Krishnan, J. et al. Activation of a HIF1α–PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 9, 512–524 (2009).
Percy, M. J. et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc. Natl Acad. Sci. USA 103, 654–659 (2006).
Percy, M. J. et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N. Engl. J. Med. 358, 162–168 (2008). This study provides evidence that HIF2α is a transcription factor that regulates EPO levels in humans.
Ang, S. O. et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nature Genet. 32, 614–621 (2002).
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nature Rev. Mol. Cell Biol. 5, 343–354 (2004).
Cao, R., Jensen, L. D., Soll, I., Hauptmann, G. & Cao, Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE 3, e2748 (2008).
Marques, I. J. et al. Transcriptome analysis of the response to chronic constant hypoxia in zebrafish hearts. J. Comp. Physiol. B 178, 77–92 (2008).
Ton, C., Stamatiou, D. & Liew, C. C. Gene expression profile of zebrafish exposed to hypoxia during development. Physiol. Genomics 13, 97–106 (2003).
van Rooijen, E. et al. Zebrafish mutants in the von Hippel–Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood 113, 6449–6460 (2009).
Azad, P., Zhou, D., Russo, E. & Haddad, G. G. Distinct mechanisms underlying tolerance to intermittent and constant hypoxia in Drosophila melanogaster. PLoS ONE 4, e5371 (2009).
Zhou, D. et al. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 4, e1000221 (2008).
Zhou, D. et al. Experimental selection for Drosophila survival in extremely low O2 environment. PLoS ONE 2, e490 (2007).
Shen, C., Nettleton, D., Jiang, M., Kim, S. K. & Powell-Coffman, J. A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 280, 20580–20588 (2005).
Bishop, T. et al. Genetic analysis of pathways regulated by the von Hippel–Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2, e289 (2004).
Kundaje, A. et al. A predictive model of the oxygen and heme regulatory network in yeast. PLoS Comput. Biol. 4, e1000224 (2008).
Hickman, M. J. & Winston, F. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell. Biol. 27, 7414–7424 (2007).
Rustad, T. R., Harrell, M. I., Liao, R. & Sherman, D. R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 3, e1502 (2008).
Park, H. D. et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48, 833–843 (2003).
Sherman, D. R. et al. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl Acad. Sci. USA 98, 7534–7539 (2001).
Baena-Gonzalez, E., Rolland, F., Thevelein, J. M. & Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942 (2007).
Loreti, E., Poggi, A., Novi, G., Alpi, A. & Perata, P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 137, 1130–1138 (2005).
Chi, J. T. et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 3, e47 (2006).
Benita, Y. et al. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 37, 4587–4602 (2009).
Chua, S.-W. et al. A novel normalization method for effective removal of systematic variation in microarray data. Nucleic Acids Res. 34, e38 (2006).
Xia, X. et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc. Natl Acad. Sci. USA 106, 4260–4265 (2009).
Pollard, P. J. et al. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1α. Biochem. J. 416, 387–394 (2008).
Beyer, S., Kristensen, M. M., Jensen, K. S., Johansen, J. V. & Staller, P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J. Biol. Chem. 283, 36542–3 6552.
Vengellur, A., Phillips, J. M., Hogenesch, J. B. & LaPres, J. J. Gene expression profiling of hypoxia signaling in human hepatocellular carcinoma cells. Physiol. Genomics 22, 308–318 (2005).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Farh, K. K. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science 310, 1817–1821 (2005).
He, L. & Hannon, G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nature Rev. Genet. 5, 522–531 (2004).
Kulshreshtha, R. et al. A microRNA signature of hypoxia. Mol. Cell. Biol. 27, 1859–1867 (2006).
Hua, Z. et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE 1, e116 (2006).
Crosby, M. E., Kulshreshtha, R., Ivan, M. & Glazer, P. M. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 69, 1221–1229 (2009).
Goldberg, A. D., Allis, C. D. & Bernstein, E. Epigenetics: a landscape takes shape. Cell 128, 635–638 (2007).
Ruthenburg, A. J., Li, H., Patel, D. J. & Allis, C. D. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nature Struct. Mol. Biol. 14, 1025–1040 (2007).
Cloos, P. A. et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 442, 307–311 (2006).
Whetstine, J. R. et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125, 467–481 (2006).
Yamane, K. et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125, 483–495 (2006).
Loenarz, C. & Schofield, C. J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nature Chem. Biol. 4, 152–156 (2008).
Holmquist-Mengelbier, L. et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell 10, 413–423 (2006). This paper shows distinct properties of HIF1α and HIF2α in acute and chronic hypoxia, respectively, and links HIF2α to tumour malignancy.
Raval, R. R. et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 25, 5675–5686 (2005).
Koshiji, M. et al. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 23, 1949–1956 (2004).
Gordan, J. D., Bertout, J. A., Hu, C. J., Diehl, J. A. & Simon, M. C. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11, 335–347 (2007).
Covello, K. L. et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 21, 1037–1049 (2006).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 7, 205–213 (1998).
Makino, Y. et al. Transcriptional up-regulation of inhibitory PAS domain protein gene expression by hypoxia-inducible factor 1 (HIF-1): a negative feedback regulatory circuit in HIF-1-mediated signaling in hypoxic cells. J. Biol. Chem. 282, 14073–14082 (2007).
Tanaka, T., Wiesener, M. S., Bernhardt, W., Eckardt, K. U. & Warnecke, C. The human hypoxia-inducible factor (HIF)-3α gene is a HIF-1 target and may modulate hypoxic gene induction. Biochem. J. 21 Aug 2009 (doi:10.1042/BJ20090120).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J. Biol. Chem. 277, 32405–3 2408.
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Webby, C. J. et al. Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science 325, 90–93 (2009). This study establishes a role of a Jumonji-domain dioxygenase in regulation of splicing and provides a possible mode by which oxygen regulates this process.
Bilton, R., Trottier, E., Pouysségur, J. & Brahimi-Horn, M. C. ARDent about acetylation and deacetylation in hypoxia signalling. Trends Cell Biol. 16, 616–621 (2006).
Dioum, E. M. et al. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science 324, 1289–1293 (2009). This paper identifies a role of the environmental stress-inducible deacetylase SIRT1 in the regulation of HIFα function.
Yamamoto, H., Schoonjans, K. & Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol. 21, 1745–1755 (2007).
Yang, X. J. & Seto, E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nature Rev. Mol. Cell Biol. 9, 206–218 (2008).
Hurlbut, G. D., Kankel, M. W., Lake, R. J. & Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19, 166–175 (2007).
Chitalia, V. C. et al. Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nature Cell Biol. 10, 1208–1216.
Wouters, B. G. & Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Rev. Cancer 8, 851–864 (2008).
Ao, A., Wang, H., Kamarajugadda, S. & Lu, J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc. Natl Acad. Sci. USA 105, 7821–7826 (2008).
Arany Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451, 1008–1012 (2008). This study describes the surprising finding that PGC1α induces VEGFA expression and angiogenesis not via the canonical hypoxia response pathway but via the orphan nuclear receptor ERRα.
Adhikary, S. & Eilers, M. Transcriptional regulation and transformation by Myc proteins. Nature Rev. Mol. Cell Biol. 6, 635–645 (2005).
Koshiji, M. et al. HIF-1α induces genetic instability by transcriptionally downregulating MutSα expression. Mol. Cell 17, 793–803 (2005).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Löfstedt, T. et al. HIF-1α induces MXI1 by alternate promoter usage in human neuroblastoma cells. Exp. Cell Res. 315, 1924–1936 (2009).
Schreiber-Agus, N. et al. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell 80, 777–786 (1995).
Gordan, J. D. et al. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14, 435–446 (2008).
Li, J. L. et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 67, 11244–11253 (2007).
Diez, H. et al. Hypoxia-mediated activation of Dll4–Notch–Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp. Cell Res. 313, 1–9 (2007).
Sahlgren, C., Gustafsson, M. V., Jin, S., Poellinger, L. & Lendahl, U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl Acad. Sci USA 105, 6392–6397 (2008).
Gustafsson, M. V. et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005). This paper provides a mechanism for how hypoxia impacts differentiation.
Bertout, J. A. et al. Heterozygosity for hypoxia inducible factor 1α decreases the incidence of thymic lymphomas in a p53 mutant mouse model. Cancer Res. 69, 3213–3220 (2009).
Coleman, M. L. et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J. Biol. Chem. 282, 24027–24038 (2007).
Zheng, X. et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl Acad. Sci. USA 105, 3368–3373 (2008).
Wilkins, S. E. et al. Differences in hydroxylation and binding of Notch and HIF-1α demonstrate substrate selectivity for factor inhibiting HIF-1 (FIH-1). Int. J. Biochem. Cell Biol. 41, 1563–1571 (2009).
Cockman, M. E., Webb, J. D., Kramer, H. B., Kessler, B. M. & Ratcliffe, P. J. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol. Cell. Proteomics 8, 535–546 (2009).
Tien, A. C. et al. Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12–Notch repeats in Drosophila melanogaster. J. Cell Biol. 182, 1113–1125 (2008).
Prasad, S. M. et al. Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif. 42, 63–74 (2009).
Jögi, A. et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl Acad. Sci. USA 99, 7021–7026 (2002).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Xie, L. et al. Oxygen-regulated β2-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL. Sci. Signal. 2, ra33 (2009). This study identifies a substrate for PHDs that is distinct from the HIFα proteins.
Cook, K. M. et al. Epidithiodiketopiperazines block the interaction between hypoxia inducible factor-1α (HIF-1α) and p300 by a zinc ejection mechanism. J. Biol. Chem. 284, 26831–26838 (2009).
Acknowledgements
We apologize that we are unable to discuss all observations that are relevant to the modulation of HIF function owing to space limitations. This work was supported by the Swedish Research Council, the Swedish Cancer Society, the European Union, the Singapore National Research Foundation and the Singapore Ministry of Education under the Research Center of Excellence Programme.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Oxidative phosphorylation
-
An aerobic, oxygen-dependent process in which electrons are transferred from electron donors to acceptors through a series of redox reactions in mitochondria. The resulting proton gradient across the inner mitochondrial membrane is used to make ATP from NADH produced, for example, by the citric acid cycle or glycolysis.
- Glycolysis
-
The metabolic pathway that converts glucose to pyruvate, which is accompanied by energy generation in the form of ATP and NADH. Monosaccharides, such as fructose and galactose, can also be metabolized in the glycolytic pathway. Under anaerobic conditions, pyruvate can be further processed to lactate. Glycolysis produces considerably less ATP per metabolized glucose molecule than oxidative phosphorylation.
- Familial erythrocytosis
-
Erythrocytosis is an increase in the number of red blood cells caused, for example, by emphysema, heart failure or respiratory diseases. The familial form is a rare inherited disorder in which the increase in red blood cells is not accompanied by an increase in white blood cells.
- Chuvash polycythaemia
-
A disease in which excess numbers of red blood cells are formed, which is often associated with elevated haematocrit levels. Some forms of polycythaemia are caused by defects in the bone marrow or increased erythropoietin production, whereas the familial form, Chuvash polycythaemia, is caused by mutations in the von Hippel–Lindau protein.
- Gluconeogenesis
-
A metabolic pathway that is used to maintain cellular or blood glucose levels and to avoid hypoglycaemia. In gluconeogenesis, glucose is generated from non-carbohydrates, such as lactate, glycerol and amino acids.
- Warburg effect
-
The observation by Warburg in the 1920s that, in contrast to normal differentiated cells, which rely primarily on mitochondrial oxidative phosphorylation to generate energy, tumour cells exhibit increased aerobic glycolysis and use of glucose. Although aerobic glycolysis was initially proposed by Warburg to be due to mitochondrial impairment, recent studies have shown a preferential switch to glycolysis in tumour cells with functional mitochondria.
Rights and permissions
About this article
Cite this article
Lendahl, U., Lee, K., Yang, H. et al. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet 10, 821–832 (2009). https://doi.org/10.1038/nrg2665
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2665
This article is cited by
-
Is there a role for [18F]-FMISO PET to guide dose adaptive radiotherapy in head and neck cancer? A review of the literature
Clinical and Translational Imaging (2024)
-
Prognostic biomarkers for the response to the radiosensitizer nimorazole combined with RCTx: a pre-clinical trial in HNSCC xenografts
Journal of Translational Medicine (2023)
-
Development and validation of a hypoxia-associated signature for lung adenocarcinoma
Scientific Reports (2022)
-
Hypoxia Conditioning for High-Altitude Pre-acclimatization
Journal of Science in Sport and Exercise (2022)
-
MYD88 signals induce tumour-initiating cell generation through the NF-κB-HIF-1α activation cascade
Scientific Reports (2021)