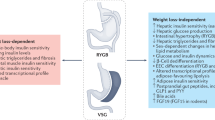
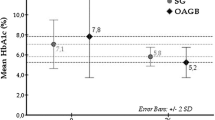
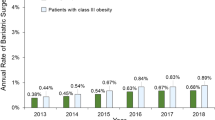
Abstract
Patients with type 2 diabetes mellitus (T2DM) are usually treated with pharmacologic agents in combination with lifestyle modification. The development of new antidiabetic agents, such as insulin analogs and incretin-based therapies, has led to treatment strategies that enable many patients with T2DM to achieve target HbA1c levels (≤7.0%). However, many factors—including those related to the patient or the health-care provider, drug inadequacies and adverse effects—can interfere with the ability of some patients to reach metabolic targets. Clinical data from the USA indicate that HbA1c concentration, blood pressure and serum levels of lipids in patients with T2DM are progressively decreasing toward the target goals set by the American Diabetes Association. These improvements in metabolic regulation have led to a 30–40% decrease in reported microvascular and macrovascular complications of diabetes mellitus in the USA. Gastric bypass surgery in morbidly obese individuals with T2DM leads to remission of the diabetes mellitus in the majority of patients and improvement in the rest. A major contributor to this improvement is an alteration in gastrointestinal hormone secretions. Interventional surgery might, therefore, be considered a reasonable therapeutic alternative for overweight and obese (BMI <35 kg/m²) patients with T2DM who do not respond to medical therapy.
Key Points
-
Lifestyle modification in combination with current pharmacologic therapies can achieve targets of metabolic control in many patients with type 2 diabetes mellitus (T2DM)
-
Factors related to the patient, health-care provider and drug regime can interfere with patients reaching these targets
-
In the USA, control of HbA1c, blood pressure and lipid values improved during 1988–1994, and were reflected in 30–40% decreases in vascular complications in patients with T2DM
-
Gastric bypass surgery in morbidly obese patients with T2DM causes remission of their diabetes mellitus or marked improvement in hyperglycemia within several weeks of the surgery
-
The mechanisms by which gastric bypass surgery improves diabetes mellitus include dramatic alterations in gastrointestinal hormone secretory patterns
-
Long-term efficacy and safety data from comparative studies are needed to evaluate gastric bypass surgery as a primary therapy for patients with T2DM and a BMI <35 kg/m2
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
DeFronzo, R. A. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Buchwald, H. et al. Weight and type 2 diabetes after bariatric surgery: systemic review and meta-analysis. Am. J. Med. 122, 248–256 (2009).
Pories, W. J. Bariatric surgery: risks and rewards. J. Clin. Endocrinol. Metab. 93 (Suppl. 1), S89–S96 (2008).
Laferrère, B. et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 93, 2479–2485 (2008).
Rubino, F., Schauer, P. R., Kaplan, L. M. & Cummings, D. E. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med. 61, 393–411 (2010).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes. Progressive requirement for multiple therapies (UKPDS 49). JAMA 281, 2005–2012 (1999).
Nathan, D. M. et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32, 193–203 (2009).
[No authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study Group. Lancet 352, 837–853 (1998).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2007).
[No authors listed] UK prospective diabetes study 16. Overview of 6 years therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 44, 1249–1258 (1995).
Ferrannini, E. et al. β-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab. 90, 493–500 (2005).
Marchetti, P., Dotta, F., Lauro, D. & Purrello, F. An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul. Pept. 146, 4–11 (2008).
Yki-Järvinen, H. et al. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann. Intern. Med. 30, 389–396 (1999).
Yki-Järvinen, H. Combination therapies with insulin in type 2 diabetes. Diabetes Care 24, 758–767 (2001).
Riddle, M. C., Rosenstock, J. & Gerich, J. The treat-to-target trial. Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26, 3080–3086 (2003).
Lebovitz, H. E. in Diabetes Mellitus: A Fundamental and Clinical Text 3rd edn Ch. 76 (eds LeRoith, D., Taylor, S. I. & Olefsky, J. R.) 1107–1122 (Lippincott Williams & Wilkins, Philadelphia, PA, 2004).
Hundal, R. S. & Inzucchi, S. E. Metformin: new understandings, new uses. Drugs 63, 1879–1894 (2003).
Lebovitz, H. E. Adjunct therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6, 326–334 (2010).
Hanefeld, M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc. Diabetol. 6, 20 (2007).
Holman, R. R. et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N. Engl. J. Med. 357, 1716–1730 (2007).
Heine, R. J. et al. Exenatide versus glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann. Intern. Med. 143, 559–569 (2005).
Drucker, D. J. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30, 1335–1343 (2007).
Lebovitz, H. E. & Banerji, M. A. Treatment of insulin resistance in diabetes mellitus. Eur. J. Pharmacol. 19, 135–146 (2004).
Buse, J. B. et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374, 39–47 (2009).
Rodbard, H. W. et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr. Pract. 15, 540–559 (2009).
Holman, R. R. et al. Three year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361, 1736–1747 (2009).
Russell-Jones, D. et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomized trial. Diabetologia 52, 2046–2055 (2009).
Zinman, B. et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care 32, 1224–1230 (2009).
Garber, A. et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomized, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373, 473–481 (2009).
Nauck, M. et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32, 84–90 (2009).
Marre, M. et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet. Med. 26, 268–278 (2009).
Drucker, D. J. Exenatide once weekly versus twice daily for treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Nauck, M. A. et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 50, 259–267 (2007).
Buse, J. B. et al. DURATION-1: Exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 33, 1255–1261 (2010).
Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008).
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Hoerger, T. J., Segel, J. E., Gregg, E. J. & Saaddine, J. B. Is glycemic control improving in US adults? Diabetes Care 31, 81–86 (2008).
Hoerger, T. J. et al. Improvements in risk factor control among persons with diabetes in the United States: evidence and implications for remaining life expectancy. Diabetes Res. Clin. Pract. 86, 225–232 (2009).
[No authors listed] The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 329, 977–986 (1993).
Writing Team for the DCCT/EDIC Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy. JAMA 290, 2159–2167 (2003).
Reaven, P. D. et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in Veterans Affairs Diabetes Trial participants with lower calcified coronary atherosclerosis. Diabetes 58, 2642–2648 (2009).
Kuller, L. H. et al. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler. Thromb. Vasc. Biol. 20, 823–829 (2000).
Liew, G., Klein, R. & Wong, T. Y. The role of genetics in susceptibility to diabetic retinopathy. Int. Ophthalmol. Clin. 49, 35–52 (2009).
Gaede, P., Lund-Andersen, H., Parving, H.-H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Nathan, D. M. et al. Intensive treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
Center for Disease Control Diabetes Data and Trends 2009 [online], (2009).
Peyrot, M. et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care 28, 2673–2697 (2005).
Schmittdiel, J. A. et al. Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J. Gen. Intern. Med. 23, 588–594 (2008).
Peyrot, M., Ruben, R. R., Kruger, D. F. & Travis, L. B. Correlates of insulin injection omission. Diabetes Care 33, 240–245 (2010).
Rodondi, N. et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia and diabetes mellitus. Ann. Intern. Med. 144, 475–484 (2006).
Hayes, R. P., Fitzgerald, J. T. & Jacober, S. J. Primary care physicians beliefs about insulin initiation in patients with type 2 diabetes. Int. J. Clin. Pract. 62, 860–868 (2008).
Marrero, D. G. Overcoming patient barriers to initiating insulin therapy in type 2 diabetes mellitus. Clin. Cornerstone 8, 33–43 (2007).
Riddle, M. C. et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33, 983–990 (2010).
Bolen, S. et al. Systemic review: comparative effectiveness and safety of oral medications for type 2 diabetes. Ann. Intern. Med. 147, 386–399 (2007).
DeFronzo, R. A., Stonehouse, A. H., Han, J. & Wintle, M. E. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet. Med. 27, 309–317 (2010).
Simpson, S. H., Majumdar, S. R., Tsuyuki, R. T., Eurich, D. T. & Johnson, J. A. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes: a population-based cohort study. CMAJ 174, 169–174 (2006).
Evans, J. M., Ogston, S. A., Emslie-Smith, A. & Morris, A. D. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49, 930–936 (2006).
de Jager, J. et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomized placebo controlled trial. BMJ 340, c2181 (2010).
Rao, A. D., Kuhadiya, N., Reynolds, K. & Fonseca, V. A. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? Diabetes Care 31, 1672–1678 (2008).
Bonds, D. E. et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 340, b4909 (2010).
NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 360, 1283–1297 (2009).
Purnell, J. Q. et al. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. JAMA 280, 140–146 (1998).
Thorn, L. M. et al. Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 32, 950–952 (2009).
Vigneri, P., Frasca, F., Sciacca, L., Pandini, G. & Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 16, 1103–1123 (2009).
Currie, C. J. et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort. Lancet 375, 481–489 (2010).
Rosenstock, J., Niggli, M. & Maldonado-Lutomirsky, M. Long-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naïve patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 11, 571–578 (2009).
Lindenfeld, J. & Masoudi, F. A. Fluid retention with thiazolidinediones: does the mechanism influence the outcome? J. Am. Coll. Cardiol. 49, 1705–1707 (2007).
Home, P. D. et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomized, open-label trial. Lancet 373, 2125–2135 (2009).
Meier, C. et al. Use of thiazolidinediones and fracture risk. Arch. Intern. Med. 168, 820–825 (2008).
Parks, M. & Rosebraugh, C. Weighing risks and benefits of liraglutide—the FDA's review of a new antidiabetic therapy. N. Engl. J. Med. 362, 774–777 (2010).
Dore, D. D., Seeger, J. D. & Chan, K. A. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr. Med. Res. Opin. 25, 1019–1027 (2009).
Shishko, P. I., Kovalev, P. A., Goncharov, V. G. & Zajarny, I. U. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes 41, 1042–1049 (1992).
Ferrannini, E. & Cobelli, C. The kinetics of insulin in man. II. Role of the liver. Diabetes Metab. Rev. 3, 365–397 (1987).
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 77, 289–312 (2008).
Rosen, C. J. Revisiting the rosiglitazone story—lessons learned. N. Engl. J. Med. 363, 803–806 (2010).
Woodcock, J., Sharfstein, J. M. & Hamburg, M. Regulatory action on rosiglitazone by US Food and Drug Administration. N. Engl. J. Med. 363, 1489–1491 (2010).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Drucker, D. J. et al. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 33, 428–433 (2010).
Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357, 753–761 (2007).
Sjöström, L. et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 10, 653–662 (2009).
Vetter, M. L., Cardillo, S., Rickels, M. R. & Iqbal, N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann. Intern. Med. 150, 94–103 (2009).
Buchwald, H. et al. Bariatric surgery: a systemic review and meta-analysis. JAMA 292, 1724–1737 (2004).
Dixon, J. B. et al. Adjustable gastric banding and conventional therapy for type 2 diabetes. A randomized controlled trial. JAMA 299, 316–323 (2008).
Flum, D. R. & Dellinger, E. P. Impact of gastric bypass operation on survival: a population-based analysis. J. Am. Coll. Surg. 199, 543–551 (2004).
Sjöström, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Christou, N. V. et al. Surgery decreases long-term mortality, morbidity and health care use in morbidly obese patients. Ann. Surg. 240, 416–424 (2004).
[No authors listed] Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am. J. Clin. Nutr. 55 (2 Suppl.), 615S–619S (1992).
Buchwald, H. & Consensus Conference Panel. Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. J. Am. Coll. Surg. 200, 593–604 (2005).
Schauer, P. R. et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 238, 467–485 (2003).
Buchwald, H., Estok, R., Fahrback, K., Banel, D. & Sledge, I. Trends in mortality in bariatric surgery: a systemic review and meta-analysis. Surgery 142, 621–635 (2007).
Pories, W. J. et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes. Ann. Surg. 222, 339–352 (1995).
Kim, S. & Richards, W. O. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann. Surg. 251, 1049–1055 (2010).
Lee, W.-J. et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI >35 and <35 kg/m2. J. Gastrointest. Surg. 12, 945–952 (2008).
Tice, J. A., Kartiner, L., Walsh, J., Petersen, A. J. & Feldman, M. D. Gastric banding or bypass? A systemic review comparing the two most popular bariatric procedures. Am. J. Med. 121, 885–893 (2008).
le Roux, C. W. et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass surgery. Ann. Surg. 246, 780–785 (2007).
Pournaras, D. J. & Le Roux, C.-W. The effect of bariatric surgery on gut hormones that alter appetite. Diabetes Metab. 35, 508–512 (2009).
Pournaras, D. J. et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes. Surg. 20, 56–60 (2010).
Thaler, J. P. & Cummings, D. E. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150, 2518–2525 (2009).
MacDonald, K. G. Jr et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J. Gastrointest. Surg. 1, 213–220 (1997).
Smith, M. D. et al. Relationship between surgeon volume and adverse outcomes after RYGB in Longitudinal Assessment of Bariatric Surgery (LABS) study. Surg. Obes. Relat. Dis. 6, 118–125 (2010).
Gasteyger, C., Suter, M., Gaillard, R. C. & Giusti, V. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am. J. Clin. Nutr. 87, 1128–1133 (2008).
Ziegler, O., Sirveau, M. A., Brunaud, L., Reibel, L. & Quilliot, D. Medical follow-up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab. 35, 544–557 (2009).
Malone, M. Recommended nutritional supplements for bariatric surgery patients. Ann. Pharmacother. 42, 1851–1858 (2008).
Patti, M. E. et al. Severe hypoglycemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic hyperplasia. Diabetologia 48, 2236–2240 (2005).
Staels, B. & Fonseca, V. A. Bile acids and metabolic regulation: mechanisms and clinical response to bile acid sequestration. Diabetes Care 32 (Suppl. 2), S237–S245 (2008).
Gaziano, J. M. et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes mellitus on overall safety and cardiovascular outcomes. Diabetes Care 33, 1503–1508 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
H. E. Lebovitz has been on the Advisory Board for Amylin, American Type Culture Collection, Biocon Pharma, Indigene, Intarca Pharmaceuticals, Merck, Metacure and Poxel Pharma. He has also acted as a consultant for Amylin, AstraZeneca, Biocon Pharma, Enzymotec, GlaxoSmithKline, Metacure and Sanofi–Aventis; has been a speaker for GlaxoSmithKline; is on the Board of Directors of Metacure; and owns stocks in Merck.
Rights and permissions
About this article
Cite this article
Lebovitz, H. Type 2 diabetes mellitus—current therapies and the emergence of surgical options. Nat Rev Endocrinol 7, 408–419 (2011). https://doi.org/10.1038/nrendo.2011.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.10
This article is cited by
-
CaV1.2 and CaV1.3 channel hyperactivation in mouse islet β cells exposed to type 1 diabetic serum
Cellular and Molecular Life Sciences (2015)
-
Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: an in vivo analysis using ZDF rats
Applied Microbiology and Biotechnology (2014)
-
Efficacy and safety of combination therapy with vildagliptin and metformin versus metformin up-titration in Chinese patients with type 2 diabetes mellitus: study design and rationale of the vision study
Cardiovascular Diabetology (2013)
-
Leptin revisited: its mechanism of action and potential for treating diabetes
Nature Reviews Drug Discovery (2012)
-
Nutritional deficiencies after bariatric surgery
Nature Reviews Endocrinology (2012)