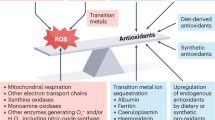
Key Points
-
Resveratrol, a small polyphenol, has been discovered and re-discovered as a potential therapeutic in recent years. Although putative cardioprotective effects were first noted in 1982, it was only after a 1992 report of high levels of resveratrol in red wine that these effects were investigated extensively. In 1997, resveratrol was isolated in a screen for cyclooxygenase inhibitors, and was shown to be an effective chemotherapeutic and chemopreventive agent. Moreover, in 2003, it was identified as the top hit in a screen for activators of sirtuin deacetylases and was shown to extend the lifespans of lower organisms.
-
The number of reported effects for resveratrol is constantly growing. Many direct targets have been identified in vitro, and protective effects have been demonstrated in various rodent models of disease.
-
Pharmacokinetic studies have consistently shown that levels of resveratrol in serum do not reach the concentrations required for most of the reported in vitro effects, or do so only transiently. In vivo evidence has therefore become increasingly important in efforts to understand how resveratrol elicits its effects in mammals.
-
One possibility that has been suggested based on data from lower organisms is that resveratrol acts as a caloric restriction mimetic. This hypothesis is intriguing because caloric restriction seems to slow the intrinsic rate of ageing, and improve general health, rather than block specific disease processes.
-
The many reported in vivo effects of resveratrol are reviewed here and, whenever possible, have been related to putative mechanisms and targets. Determining the mechanism(s) by which resveratrol and similar molecules act, and developing methods to improve bioavailability and/or specificity, has enormous potential to benefit human health.
Abstract
Resveratrol, a constituent of red wine, has long been suspected to have cardioprotective effects. Interest in this compound has been renewed in recent years, first from its identification as a chemopreventive agent for skin cancer, and subsequently from reports that it activates sirtuin deacetylases and extends the lifespans of lower organisms. Despite scepticism concerning its bioavailability, a growing body of in vivo evidence indicates that resveratrol has protective effects in rodent models of stress and disease. Here, we provide a comprehensive and critical review of the in vivo data on resveratrol, and consider its potential as a therapeutic for humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Takaoka, M. J. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.). J. Faculty Sci. Hokkaido Imperial University 3, 1–16 (1940).
Nonomura, S., Kanagawa, H. & Makimoto, A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-jo-kon (Polygonum cuspidatum Sieb. et Zucc.). Yakugaku Zasshi 83, 988–990 (1963).
Langcake, P. & Pryce, R. J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 9, 77–86 (1976).
Siemann, E. H. & Creasy, L. L. Concentration of the phytoalexin resveratrol in wine. Am. J. Eno. Vitic. 43, 49–52 (1992).
Jang, M. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 (1997). Shows that resveratrol is a naturally occurring inhibitor of cyclooxygenase that has both anti-inflammatory and anticarcinogenic properties in vivo.
Bradamante, S., Barenghi, L. & Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 22, 169–188 (2004).
Wang, Q. et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 958, 439–447 (2002).
Sinha, K., Chaudhary, G. & Gupta, Y. K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 71, 655–665 (2002).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003). The first evidence that resveratrol is an activator of sirtuin deacetylases and extends the lifespan of S. cerevisiae.
Valenzano, D. R. et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 (2006). Shows that resveratrol can extend lifespan and delay neurodegeneration in vertebrates, although sirtuin-dependence of the effect is not addressed.
Buryanovskyy, L. et al. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry 43, 11417–11426 (2004). Provides a detailed description of the binding pocket for resveratrol on the highest-affinity target reported so far, as well as a discussion of the consequences of QR2 inhibition.
Barger, J. L., Walford, R. L. & Weindruch, R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp. Gerontol. 38, 1343–1351 (2003).
McCay, C. M. & Crowell, M. F. Prolonging the lifespan. Scientific Monthly 39, 405–414 (1934).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Tessitore, L., Davit, A., Sarotto, I. & Caderni, G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis 21, 1619–1622 (2000).
Chen, Y., Tseng, S. H., Lai, H. S. & Chen, W. J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 136, 57–66 (2004).
Bove, K., Lincoln, D. W. & Tsan, M. F. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 291, 1001–1005 (2002).
Oshima, M. et al. Suppression of intestinal polyposis in Apcδ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–809 (1996).
Zha, S., Yegnasubramanian, V., Nelson, W. G., Isaacs, W. B. & De Marzo, A. M. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 215, 1–20 (2004).
Khanduja, K. L., Bhardwaj, A. & Kaushik, G. Resveratrol inhibits N-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 50, 61–65 (2004).
Li, Z. G. et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis 23, 1531–1536 (2002).
Aziz, M., Afaq, F. & Ahmad, N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem. Photobiol. 81, 25–31 (2004).
Subbaramaiah, K. et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 273, 21875–21882 (1998).
Martin, A. R., Villegas, I., La Casa, C. & de la Lastra, C. A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 67, 1399–1410 (2004).
Stewart, J. R., Ward, N. E., Ioannides, C. G. & O'Brian, C. A. Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochemistry 38, 13244–13251 (1999). Shows that the IC 50 of resveratrol against PKC is substrate- and co-factor-dependent, which could explain the disparity between effective concentrations in vitro and serum concentrations in vivo for this and other putative targets.
Schneider, Y. et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 158, 85–91 (2000).
Fu, Z. D., Cao, Y., Wang, K. F., Xu, S. F. & Han, R. Chemopreventive effect of resveratrol to cancer. Ai Zheng 23, 869–873 (2004).
Afaq, F., Adhami, V. M. & Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 186, 28–37 (2003).
Martinez, M. E. et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl Acad. Sci. USA 100, 7859–7864 (2003).
Meyskens, F. L. Jr & Gerner, E. W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. 5, 945–951 (1999).
Kimura, Y. & Okuda, H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J. Nutr. 131, 1844–1849 (2001).
Tseng, S. H. et al. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 10, 2190–2202 (2004).
Brakenhielm, E., Cao, R. & Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 15, 1798–1800 (2001).
Giudice, A. & Montella, M. Activation of the Nrf2–ARE signaling pathway: a promising strategy in cancer prevention. Bioessays 28, 169–181 (2006).
Yu, C., Shin, Y. G., Kosmeder, J. W., Pezzuto, J. M. & van Breemen, R. B. Liquid chromatography/tandem mass spectrometric determination of inhibition of human cytochrome P450 isozymes by resveratrol and resveratrol-3-sulfate. Rapid Commun. Mass Spectrom. 17, 307–313 (2003).
Piver, B., Berthou, F., Dreano, Y. & Lucas, D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 125, 83–91 (2001).
Chang, T. K., Lee, W. B. & Ko, H. H. Trans-resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen-activating human cytochrome P450 1B1. Can. J. Physiol. Pharmacol. 78, 874–881 (2000).
Chan, W. K. & Delucchi, A. B. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci. 67, 3103–3112 (2000).
Ciolino, H. P., Daschner, P. J. & Yeh, G. C. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 58, 5707–5712 (1998).
Casper, R. F. et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol. Pharmacol. 56, 784–790 (1999).
Zhou, S. et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 44, 279–304 (2005).
Revel, A. et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 23, 255–261 (2003). Describes the in vivo confirmation of effects predicted on the basis of the inhibition of the AHR by resveratrol.
Berge, G., Ovrebo, S., Eilertsen, E., Haugen, A. & Mollerup, S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br. J. Cancer 91, 1380–1383 (2004).
Cao, Z. & Li, Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur. J. Pharmacol. 489, 39–48 (2004).
Kaga, S., Zhan, L., Matsumoto, M. & Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell Cardiol. 39, 813–822 (2005).
Floreani, M., Napoli, E., Quintieri, L. & Palatini, P. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 72, 2741–2750 (2003).
Long, D. J. et al. Disruption of dihydronicotinamide riboside:quinone oxidoreductase 2 (NQO2) leads to myeloid hyperplasia of bone marrow and decreased sensitivity to menadione toxicity. J. Biol. Chem. 277, 46131–46139 (2002).
Long, D. J. et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 62, 3030–3036 (2002).
Hebbar, V. et al. Toxicogenomics of resveratrol in rat liver. Life Sci. 76, 2299–2314 (2005).
Aggarwal, B. B. et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 24, 2783–2840 (2004).
Yu, L., Sun, Z. J., Wu, S. L. & Pan, C. E. Effect of resveratrol on cell cycle proteins in murine transplantable liver cancer. World J. Gastroenterol. 9, 2341–2343 (2003).
Schneider, Y. et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer 39, 102–107 (2001).
Reagan-Shaw, S., Afaq, F., Aziz, M. H. & Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 23, 5151–5160 (2004).
Garvin, S., Ollinger, K. & Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 231, 113–122 (2006).
Provinciali, M. et al. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer 115, 36–45 (2005).
Zhou, H. B., Chen, J. J., Wang, W. X., Cai, J. T. & Du, Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J. Gastroenterol. 11, 280–284 (2005).
Gautam, S. C., Xu, Y. X., Dumaguin, M., Janakiraman, N. & Chapman, R. A. Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant. 25, 639–645 (2000).
Ferry-Dumazet, H. et al. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis 23, 1327–1333 (2002).
Fulda, S. & Debatin, K. M. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 64, 337–346 (2004).
Kensler, T. et al. Role of reactive intermediates in tumor promotion and progression. Prog. Clin. Biol. Res. 391, 103–116 (1995).
Gromadzinska, J. & Wasowicz, W. The role of reactive oxygen species in the development of malignancies. Int. J. Occup. Med. Environ. Health 13, 233–245 (2000).
Sengottuvelan, M., Viswanathan, P. & Nalini, N. Chemopreventive effect of trans-resveratrol — a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis 27, 1038–1046 (2005).
Miura, D., Miura, Y. & Yagasaki, K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 73, 1393–1400 (2003).
Wenzel, E., Soldo, T., Erbersdobler, H. & Somoza, V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 49, 482–494 (2005).
Collins, A. R. Antioxidant intervention as a route to cancer prevention. Eur. J. Cancer 41, 1923–1930 (2005).
Renaud, S. & Gueguen, R. The French paradox and wine drinking. Novartis Found. Symp. 216, 208–217, discussion 152–158, 217–222 (1998).
Renaud, S. & de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339, 1523–1526 (1992).
Richard, J. L. Coronary risk factors. The French paradox. Arch. Mal. Coeur Vaiss. 80, 17–21 (1987).
Gronbaek, M. et al. Mortality associated with moderate intakes of wine, beer, or spirits. BMJ 310, 1165–1169 (1995).
Bohm, M., Rosenkranz, S. & Laufs, U. Alcohol and red wine: impact on cardiovascular risk. Nephrol. Dial. Transplant. 19, 11–16 (2004).
Seigneur, M. et al. Effect of the consumption of alcohol, white wine, and red wine on platelet function and serum lipids. J. Appl. Cardiol. 5, 215–222 (1990).
Demrow, H. S., Slane, P. R. & Folts, J. D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation 91, 1182–1188 (1995).
Fitzpatrick, D. F., Hirschfield, S. L. & Coffey, R. G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 265, H774–H778 (1993).
Lekakis, J. et al. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Cardiovasc. Prev. Rehabil. 12, 596–600 (2005).
Wang, Z. et al. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int. J. Mol. Med. 16, 533–540 (2005).
Fuhrman, B., Lavy, A. & Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 61, 549–554 (1995).
Frankel, E. N., Kanner, J., German, J. B., Parks, E. & Kinsella, J. E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 341, 454–457 (1993).
Zern, T. L., West, K. L. & Fernandez, M. L. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. J. Nutr. 133, 2268–2272 (2003).
Bertelli, A. A. et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 17, 1–3 (1995).
Wang, Z. et al. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int. J. Mol. Med. 9, 77–79 (2002).
Zini, R., Morin, C., Bertelli, A., Bertelli, A. A. & Tillement, J. P. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp. Clin. Res. 25, 87–97 (1999).
Hamberg, M., Svensson, J. & Samuelsson, B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl Acad. Sci. USA 72, 2994–2998 (1975).
Hamberg, M., Svensson, J., Wakabayashi, T. & Samuelsson, B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc. Natl Acad. Sci. USA 71, 345–349 (1974).
Moncada, S., Gryglewski, R., Bunting, S. & Vane, J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263, 663–665 (1976).
Mukherjee, D., Nissen, S. E. & Topol, E. J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286, 954–959 (2001).
Davies, N. M. & Jamali, F. COX-2 selective inhibitors cardiac toxicity: getting to the heart of the matter. J. Pharm. Pharm. Sci. 7, 332–336 (2004).
Szewczuk, L. M., Forti, L., Stivala, L. A. & Penning, T. M. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 279, 22727–22737 (2004).
Williams, A. & Hennekens, C. H. The role of aspirin in cardiovascular diseases — forgotten benefits? Expert Opin. Pharmacother. 5, 109–115 (2004).
Vane, J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 231, 232–235 (1971).
Mitchell, J. A., Akarasereenont, P., Thiemermann, C., Flower, R. J. & Vane, J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl Acad. Sci. USA 90, 11693–11697 (1993).
Naderali, E. K., Doyle, P. J. & Williams, G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin. Sci. (Lond.) 98, 537–543 (2000).
Jager, U. & Nguyen-Duong, H. Relaxant effect of trans-resveratrol on isolated porcine coronary arteries. Arzneimittelforschung 49, 207–211 (1999).
Li, H. F., Chen, S. A. & Wu, S. N. Evidence for the stimulatory effect of resveratrol on Ca2+-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 45, 1035–1045 (2000).
Orallo, F. et al. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 61, 294–302 (2002).
Das, S. et al. Coordinated induction of iNOS–VEGF–KDR–eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul. Pharmacol. 42, 281–289 (2005). Explores the molecular basis of resveratrol's capacity to protect hearts from ischaemic injury, both upstream and downstream of nitric oxide.
Cruz, M. N. et al. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am. J. Physiol. Heart Circ. Physiol. 290, H1969–H1975 (2005).
Holvoet, P. Oxidized LDL and coronary heart disease. Acta Cardiol. 59, 479–484 (2004).
Frankel, E. N., Waterhouse, A. L. & Kinsella, J. E. Inhibition of human LDL oxidation by resveratrol. Lancet 341, 1103–1104 (1993).
Fremont, L., Belguendouz, L. & Delpal, S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 64, 2511–2521 (1999).
Turrens, J. F., Lariccia, J. & Nair, M. G. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic. Res. 27, 557–562 (1997).
Urpi-Sarda, M. et al. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal. Chem. 77, 3149–3155 (2005).
Morales, A. I. et al. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid. Redox Signal. 4, 893–898 (2002).
Mizutani, K., Ikeda, K., Kawai, Y. & Yamori, Y. Protective effect of resveratrol on oxidative damage in male and female stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 28, 55–59 (2001).
Arichi, H. et al. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem. Pharm. Bull. (Tokyo) 30, 1766–1770 (1982).
Wilson, T., Knight, T. J., Beitz, D. C., Lewis, D. S. & Engen, R. L. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 59, PL15–PL21 (1996).
Turner, R. T., Evans, G. L., Zhang, M., Maran, A. & Sibonga, J. D. Is resveratrol an estrogen agonist in growing rats? Endocrinology 140, 50–54 (1999).
Wang, Z. et al. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin. Med. J. (Engl.) 115, 378–380 (2002).
Kollar, P. et al. Experimental study of resveratrol and flavonoids in red wine with regard to their possible hypolipemic effects. Vnitr. Lek. 46, 856–860 (2000).
Irikura, T., Takagi, K., Okada, K. & Yagasaki, K. Effect of KCD-232, a new hypolipidemic agent, on serum lipoprotein changes in hepatoma-bearing rats. Lipids 20, 420–424 (1985).
Lobo, R. A. Benefits and risks of estrogen replacement therapy. Am. J. Obstet. Gynecol. 173, 982–989 (1995).
Gehm, B. D., McAndrews, J. M., Chien, P. Y. & Jameson, J. L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl Acad. Sci. USA 94, 14138–14143 (1997).
Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur. J. Endocrinol. 138, 619–620 (1998).
Hodis, H. N. et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N. Engl. J. Med. 349, 535–545 (2003).
Bluming, A. Z. Hormone replacement therapy: the debate should continue. Geriatrics 59, 30–31, 35–37 (2004).
Simmons, D. L., Botting, R. M. & Hla, T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56, 387–437 (2004).
Chen, G. et al. Synthesis and anti-inflammatory activity of resveratrol analogs. Chem. Pharm. Bull. (Tokyo) 53, 1587–1590 (2005).
Birrell, M. A. et al. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-κB-independent mechanism. FASEB J. 19, 840–841 (2005).
Elmali, N. et al. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 54, 158–162 (2005).
Wu, S. L., Pan, C. E., Yu, L. & Meng, K. W. Immunosuppression by combined use of cyclosporine and resveratrol in a rat liver transplantation model. Transplant. Proc. 37, 2354–2359 (2005).
Wu, S. L., Yu, L., Meng, K. W., Ma, Z. H. & Pan, C. E. Resveratrol prolongs allograft survival after liver transplantation in rats. World J. Gastroenterol. 11, 4745–4749 (2005).
Shigematsu, S. et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic. Biol. Med. 34, 810–817 (2003).
Suzuki, M. et al. Superoxide mediates reperfusion-induced leukocyte–endothelial cell interactions. Am. J. Physiol. 257, H1740–H1745 (1989).
Kimmey, M. B. Cardioprotective effects and gastrointestinal risks of aspirin: maintaining the delicate balance. Am. J. Med. 117 (Suppl. 5A), S72–S78 (2004).
Feng, Y. H. et al. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol. Sin. 23, 893–897 (2002).
Docherty, J. J. et al. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 67, 155–162 (2005).
Docherty, J. J., Smith, J. S., Fu, M. M., Stoner, T. & Booth, T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 61, 19–26 (2004).
Ray, P. S. et al. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 27, 160–169 (1999).
Sato, M. et al. Myocardial protection with red wine extract. J. Cardiovasc. Pharmacol. 35, 263–268 (2000).
Bradamante, S. et al. Does resveratrol induce pharmacological preconditioning? Int. J. Tissue React. 22, 1–4 (2000).
Hung, L. M. et al. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br. J. Pharmacol. 135, 1627–1633 (2002).
Hattori, R., Otani, H., Maulik, N. & Das, D. K. Pharmacological preconditioning with resveratrol: role of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 282, H1988–H1995 (2002).
Imamura, G. et al. Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am. J. Physiol. Heart Circ. Physiol. 282, H1996–H2003 (2002).
Hung, L. M., Chen, J. K., Huang, S. S., Lee, R. S. & Su, M. J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 47, 549–555 (2000).
Bradamante, S. et al. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur. J. Pharmacol. 465, 115–123 (2003).
Inoue, H. et al. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor α in mice. Neurosci. Lett. 352, 203–206 (2003).
Wang, Y. J., He, F. & Li, X. L. The neuroprotection of resveratrol in the experimental cerebral ischemia. Zhonghua Yi Xue Za Zhi 83, 534–536 (2003).
Gupta, Y. K., Chaudhary, G., Sinha, K. & Srivastava, A. K. Protective effect of resveratrol against intracortical FeCl3-induced model of posttraumatic seizures in rats. Methods Find. Exp. Clin. Pharmacol. 23, 241–244 (2001).
Gupta, Y. K., Briyal, S. & Chaudhary, G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol. Biochem. Behav. 71, 245–249 (2002).
Gupta, Y. K., Chaudhary, G. & Srivastava, A. K. Protective effect of resveratrol against pentylenetetrazole-induced seizures and its modulation by an adenosinergic system. Pharmacology 65, 170–174 (2002).
Sharma, M. & Gupta, Y. K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 71, 2489–2498 (2002).
Liu, Z., Yu, B., Li, W. & Sun, J. Estrogenicity of trans-resveratrol in immature mice in vivo. Wei Sheng Yan Jiu 31, 188–190 (2002).
Freyberger, A., Hartmann, E., Hildebrand, H. & Krotlinger, F. Differential response of immature rat uterine tissue to ethinylestradiol and the red wine constituent resveratrol. Arch. Toxicol. 74, 709–715 (2001).
Kubo, K. et al. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 45, 345–356 (2003).
Kyselova, V., Peknicova, J., Buckiova, D. & Boubelik, M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD-1 mice. Reprod. Biol. Endocrinol. 1, 30 (2003).
Nikaido, Y. et al. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 18, 803–811 (2004).
Juan, M. E., Vinardell, M. P. & Planas, J. M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 132, 257–260 (2002).
Crowell, J. A., Korytko, P. J., Morrissey, R. L., Booth, T. D. & Levine, B. S. Resveratrol-associated renal toxicity. Toxicol. Sci. 82, 614–619 (2004).
Gentilli, M. et al. Resveratrol decreases hyperalgesia induced by carrageenan in the rat hind paw. Life Sci. 68, 1317–1321 (2001).
Torres-Lopez, J. E. et al. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 70, 1669–1676 (2002).
Granados-Soto, V., Arguelles, C. F. & Ortiz, M. I. The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology 43, 917–923 (2002).
Seidman, M., Babu, S., Tang, W., Naem, E. & Quirk, W. S. Effects of resveratrol on acoustic trauma. Otolaryngol. Head Neck Surg. 129, 463–470 (2003).
Lugarini, F., Hrupka, B. J., Schwartz, G. J., Plata-Salaman, C. R. & Langhans, W. A role for cyclooxygenase-2 in lipopolysaccharide-induced anorexia in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R862–R868 (2002).
Cadenas, S. & Barja, G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3 . Free Radic. Biol. Med. 26, 1531–1537 (1999).
Giovannini, L. et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J. Cardiovasc. Pharmacol. 37, 262–270 (2001).
Yang, Y. & Piao, Y. Effects of resveratrol on Ca2+, Mg2+-ATPase activities after spinal cord trauma in rats. Zhong Yao Cai 25, 882–885 (2002).
Yang, Y. B. & Piao, Y. J. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol. Sin. 24, 703–710 (2003).
Fulgenzi, A., Bertelli, A. A., Magni, E., Ferrero, E. & Ferrero, M. E. In vivo inhibition of TNFα-induced vascular permeability by resveratrol. Transplant. Proc. 33, 2341–2343 (2001).
McClintock, S. D., Till, G. O., Smith, M. G. & Ward, P. A. Protection from half-mustard-gas-induced acute lung injury in the rat. J. Appl. Toxicol. 22, 257–262 (2002).
Korolkiewicz, R. P. et al. Differential salutary effects of nonselective and selective COX-2 inhibitors in postoperative ileus in rats. J. Surg. Res. 109, 161–169 (2003).
Korolkiewicz, R. P. et al. The role and interactions of nitric oxide (NO), carbon monoxide (CO), and prostanoids in the pathogenesis of postoperative ileus in rats. J. Gastrointest. Surg. 8, 346–357 (2004).
Brachmann, C. B. et al. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 9, 2888–2902 (1995).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004).
McBurney, M. W. et al. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 23, 38–54 (2003).
Guarente, L. & Picard, F. Calorie restriction — the SIR2 connection. Cell 120, 473–482 (2005).
Koubova, J. & Guarente, L. How does calorie restriction work? Genes Dev. 17, 313–321 (2003).
Kaeberlein, M. et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 280, 17038–17045 (2005).
Borra, M. T., Smith, B. C. & Denu, J. M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 280, 17187–17195 (2005).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Yeung, F. et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Parker, J. A. et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genet. 37, 349–350 (2005).
Viswanathan, M., Kim, S. K., Berdichevsky, A. & Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 9, 605–615 (2005). Shows that activation of SIR-2.1 (a C. elegans sirtuin deacetylase) by resveratrol extends lifespan in a manner that is distinct from simple overexpression.
Marier, J. F. et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 302, 369–373 (2002).
Asensi, M. et al. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 33, 387–398 (2002).
Walle, T., Hsieh, F., DeLegge, M. H., Oatis, J. E. Jr & Walle, U. K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 32, 1377–1382 (2004). The most detailed description so far of resveratrol metabolism in humans.
Vitaglione, P. et al. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 49, 495–504 (2005).
Gescher, A. J. & Steward, W. P. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol. Biomarkers Prev. 12, 953–957 (2003).
Pervaiz, S. Resveratrol: from grapevines to mammalian biology. FASEB J. 17, 1975–1985 (2003).
Soleas, G. J., Diamandis, E. P. & Goldberg, D. M. Resveratrol: a molecule whose time has come? And gone? Clin. Biochem. 30, 91–113 (1997).
Mukamal, K. J. et al. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA 289, 1405–1413 (2003).
Cleophas, T. J. Wine, beer and spirits and the risk of myocardial infarction: a systematic review. Biomed. Pharmacother. 53, 417–423 (1999).
De Santi, C., Pietrabissa, A., Spisni, R., Mosca, F. & Pacifici, G. M. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica 30, 609–617 (2000).
Sale, S. et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer 90, 736–744 (2004).
Vitrac, X. et al. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 72, 2219–2233 (2003).
Mertens-Talcott, S. U. & Percival, S. S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 218, 141–151 (2005).
Chan, M. M., Mattiacci, J. A., Hwang, H. S., Shah, A. & Fong, D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 60, 1539–1548 (2000).
Fang, J. G. et al. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chemistry 8, 4191–4198 (2002).
Conte, A., Pellegrini, S. & Tagliazucchi, D. Synergistic protection of PC12 cells from β-amyloid toxicity by resveratrol and catechin. Brain Res. Bull. 62, 29–38 (2003).
Heredia, A., Davis, C. & Redfield, R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J. Acquir. Immune Defic. Syndr. 25, 246–255 (2000).
Su, H. C., Hung, L. M. & Chen, J. K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 24 Jan 2006 [epub ahead of print]. Reports that resveratrol causes a significant improvement of the phenotype in a rat model of diabetes, improving glucose and triglyceride metabolism, and preventing overeating.
Roth, G. S., Lane, M. A. & Ingram, D. K. Caloric restriction mimetics: the next phase. Ann. NY Acad. Sci. 1057, 365–371 (2005).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Lopez-Lluch, G. et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl Acad. Sci. USA 103, 1768–1773 (2006).
Storz, P., Doppler, H. & Toker, A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor-κB. Mol. Pharmacol. 66, 870–879 (2004).
Lamming, D. W., Wood, J. G. & Sinclair, D. A. Small molecules that regulate lifespan: evidence for xenohormesis. Mol. Microbiol. 53, 1003–1009 (2004).
Sinclair, D. A. & Howitz, K. T. in Handbook of the Biology of Aging (eds Masoro, E. J. & Austad, S. N.) 63–104 (Elsevier, Boston, 2006).
Lane, M. A., Ingram, D. K. & Roth, G. S. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of caloric restriction. J. Anti-Aging Med. 1, 327–337 (1998).
Calabrese, E. J. Hormesis: from marginalization to mainstream: a case for hormesis as the default dose-response model in risk assessment. Toxicol. Appl. Pharmacol. 197, 125–136 (2004).
Rattan, S. I. Aging, anti-aging, and hormesis. Mech. Ageing Dev. 125, 285–289 (2004).
Mine, M., Okumura, Y., Ichimaru, M., Nakamura, T. & Kondo, S. Apparently beneficial effect of low to intermediate doses of A-bomb radiation on human lifespan. Int. J. Radiat. Biol. 58, 1035–1043 (1990).
Masoro, E. J. Hormesis and the antiaging action of dietary restriction. Exp. Gerontol. 33, 61–66 (1998).
Chippindale, A. K., Leroi, A. M., Kim, S. B. & Rose, M. R. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J. Evolutionary Biol. 6, 171–193 (1993).
Anderson, R. M., Bitterman, K. J., Wood, J. G., Medvedik, O. & Sinclair, D. A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423, 181–185 (2003).
Bauer, J. H., Goupil, S., Garber, G. B. & Helfand, S. L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 101, 12980–12985 (2004).
Goldberg, D. M. et al. A global survey of trans-resveratrol concentrations in commercial wines. Am. J. Enol. Vitic. 46, 159–165 (1995).
Careri, M., Corradini, C., Elviri, L., Nicoletti, I. & Zagnoni, I. Liquid chromatography-electrospray tandem mass spectrometry of cis-resveratrol and trans-resveratrol: development, validation, and application of the method to red wine, grape, and winemaking byproducts. J. Agric. Food Chem. 52, 6868–6874 (2004).
Kiraly-Veghely, Z., Tyihak, E., Albert, L., Nemeth, Z. I. & Katay, G. Identification and measurement of resveratrol and formaldehyde in parts of white and blue grape berries. Acta Biol. Hung. 49, 281–289 (1998).
Ribeiro de Lima, M. T. et al. Determination of stilbenes (trans-astringin, cis- and trans-piceid, and cis- and trans-resveratrol) in Portuguese wines. J. Agric. Food Chem. 47, 2666–2670 (1999).
Burns, J., Yokota, T., Ashihara, H., Lean, M. E. & Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 50, 3337–3340 (2002).
Mark, L., Nikfardjam, M. S., Avar, P. & Ohmacht, R. A validated HPLC method for the quantitative analysis of trans-resveratrol and trans-piceid in Hungarian wines. J. Chromatogr. Sci. 43, 445–449 (2005).
Vitrac, X. et al. Determination of stilbenes (δ-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, ɛ-viniferin) in Brazilian wines. J. Agric. Food Chem. 53, 5664–5669 (2005).
Soleas, G. J. et al. A derivatized gas chromatographic-mass spectrometric method for the analysis of both isomers of resveratrol in juice and wine. Am. J. Enol. Vitic. 46, 346–352 (1995).
Creasy, L. L. & Coffee, M. Phytoalexin production potential of grape berries. J. Am. Soc. Hortic. Sci. 113, 230–234 (1988).
Rimando, A. M., Kalt, W., Magee, J. B., Dewey, J. & Ballington, J. R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 52, 4713–4719 (2004).
Romero-Perez, A. I., Lamuela-Raventos, R. M., Andres-Lacueva, C. & de La Torre-Boronat, M. C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 49, 210–215 (2001).
Romero-Perez, A. I., Ibern-Gomez, M., Lamuela-Raventos, R. M. & de La Torre-Boronat, M. C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 47, 1533–1536 (1999).
Wang, Y., Catana, F., Yang, Y., Roderick, R. & van Breemen, R. B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 50, 431–435 (2002).
Lyons, M. M. et al. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 51, 5867–5870 (2003).
Sanders, T. H., McMichael, R. W. Jr & Hendrix, K. W. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 48, 1243–1246 (2000).
Tokusoglu, O., Unal, M. K. & Yemis, F. Determination of the phytoalexin resveratrol (3,5,4′-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography-mass spectrometry (GC-MS). J. Agric. Food Chem. 53, 5003–5009 (2005).
Sobolev, V. S. & Cole, R. J. trans-Resveratrol content in commercial peanuts and peanut products. J. Agric. Food Chem. 47, 1435–1439 (1999).
Ibern-Gomez, M., Roig-Perez, S., Lamuela-Raventos, R. M. & de la Torre-Boronat, M. C. Resveratrol and piceid levels in natural and blended peanut butters. J. Agric. Food Chem. 48, 6352–6354 (2000).
Ingham, J. L. 3,5,4′-Trihydroxystilbene as a phytoalexin from groundnuts (Arachis hypogaea). Phytochemistry 15, 1791–1793 (1976).
Matsuda, H. et al. Effects of stilbene constituents from rhubarb on nitric oxide production in lipopolysaccharide-activated macrophages. Bioorg. Med. Chem. Lett. 10, 323–327 (2000).
Callemien, D., Jerkovic, V., Rozenberg, R. & Collin, S. Hop as an interesting source of resveratrol for brewers: optimization of the extraction and quantitative study by liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. J. Agric. Food Chem. 53, 424–429 (2005).
Jerkovic, V., Callemien, D. & Collin, S. Determination of stilbenes in hop pellets from different cultivars. J. Agric. Food Chem. 53, 4202–4206 (2005).
Ali, Z. et al. Phenolic constituents of Gnetum klossii. J. Nat. Prod. 66, 558–560 (2003).
Aaviksaar, A. et al. Hydroxystilbenes in the roots of Rheum rhaponticum. Proc. Estonian Acad. Sci. Chem. 52, 99–107 (2003).
Olas, B., Wachowicz, B., Stochmal, A. & Oleszek, W. Anti-platelet effects of different phenolic compounds from Yucca schidigera Roezl. bark. Platelets 13, 167–173 (2002).
Chen, R. S., Wu, P. L. & Chiou, R. Y. Peanut roots as a source of resveratrol. J. Agric. Food Chem. 50, 1665–1667 (2002).
Powell, R. G., TePaske, M. R., Plattner, R. D., White, J. F. & Clement, S. L. Isolation of resveratrol from Festuca versuta and evidence for the widespread occurrence of this stilbene in the Poaceae. Phytochemistry 35, 335–338 (1994).
Kumar, R. J., Jyostna, D., Krupadanam, G. L. D. & Srimannarayana, G. Phenanthrene and stilbenes from Pterolobium hexapetullum. Phytochemistry 27, 3625–3626 (1988).
Rolfs, C. & Kindl, H. Two different constitutive enzymes in cultured cells of Picea excelsa. Plant Physiol. 75, 489–492 (1984).
Hathaway, D. W. & Seakins, J. W. T. Hydroxystilbenes of Eucalyptus wandoo. Biochem. J. 72, 369–374 (1959).
Deshpande, V. H., Srinivasan, R. & Rao, A. V. Wood phenolics of Morus species. IV. Phenolics of the heartwood of five Morus species. Indian J. Chem. 13, 453–457 (1975).
Anjaneyulu, A. S. R. et al. A new dibenzo (2,3-6,7) oxepin derivative from Bauhinia racemosa. Tetrahedron 40, 4245–4252 (1984).
Cantos, E., Garcia-Viguera, C., de Pascual-Teresa, S. & Tomas-Barberan, F. A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 48, 4606–4612 (2000).
Cantos, E., Espin, J. C. & Tomas-Barberan, F. A. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: a new 'functional' fruit? J. Agric. Food Chem. 49, 5052–5058 (2001).
Cantos, E., Espin, J. C. & Tomas-Barberan, F. A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 50, 6322–6329 (2002).
Juan, M. E., Buenafuente, J., Casals, I. & Planas, J. M. Plasmatic levels of trans-resveratrol in rats. Food Res. Int. 35, 195–199 (2002).
Meng, X., Maliakal, P., Lu, H., Lee, M. J. & Yang, C. S. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 52, 935–942 (2004).
Juan, M. E., Lamuela-Raventos, R. M., de la Torre-Boronat, M. C. & Planas, J. M. Determination of trans-resveratrol in plasma by HPLC. Anal. Chem. 71, 747–750 (1999).
Yu, C. et al. Human, rat, and mouse metabolism of resveratrol. Pharm. Res. 19, 1907–1914 (2002).
Goldberg, D. M., Yan, J. & Soleas, G. J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 36, 79–87 (2003).
Bertelli, A. A., Giovannini, L., Stradi, R., Bertelli, A. & Tillement, J. P. Plasma, urine and tissue levels of trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. Int. J. Tissue React. 18, 67–71 (1996).
Bertelli, A., Bertelli, A. A., Gozzini, A. & Giovannini, L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp. Clin. Res. 24, 133–138 (1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.A.S. and J.A.B. are inventors on provisional patents for the use of resveratrol in treating age-associated diseases.
D.A.S. is a co-founder of and consultant to Sirtris, a company whose goal is to treat diseases of ageing by activating sirtuins.
Research in the laboratory of D.A.S. is not funded by industry.
Supplementary information
Supplementary information S1 (table)
(PDF 98 kb)
Glossary
- Phytoalexin
-
A toxic compound produced by higher plants in response to infection or other stresses, such as nutrient deprivation.
- Caloric restriction
-
A reduction of calorie intake (typically by 30–40% in rodents) to a level that does not cause malnutritionand that has been shown to increase lifespan and stress-resistance in multiple species.
- Sirtuin
-
A member of the family of NAD+-dependent deacetylases named after the Saccharomyces cerevisiae silent information regulator 2 (Sir2) protein (class III histone deacetylases).
Rights and permissions
About this article
Cite this article
Baur, J., Sinclair, D. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5, 493–506 (2006). https://doi.org/10.1038/nrd2060
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd2060
This article is cited by
-
Oxyresveratrol attenuates bone resorption by inhibiting the mitogen-activated protein kinase pathway in ovariectomized rats
Nutrition & Metabolism (2024)
-
Bio synthesis, comprehensive characterization, and multifaceted therapeutic applications of BSA-Resveratrol coated platinum nanoparticles
Scientific Reports (2024)
-
Resveratrol impacts on aquatic animals: a review
Fish Physiology and Biochemistry (2024)
-
Accelerated wound healing with resveratrol‐loaded decellularized pericardium in mice model
Cell and Tissue Banking (2024)
-
Synergistic inhibitory actions of resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: a combinational drug approach
Medical Oncology (2024)