Key Points
-
Solution-phase synthesis is a slow process due to the need for iterative coupling and deprotection steps, with purification at each step along the way. The first automated solid-phase oligosaccharide synthesizer based on a modified peptide synthesizer was introduced in 2001.
-
A number of highly successful drugs based on carbohydrates have been developed, such as acarbose, an α-glucosidase inhibitor, for the treatment of diabetes mellitus. Heparin is an even older carbohydrate-based drug that has been used since the 1940s. Much effort has been put in to preparing highly active synthetic heparin structures.
-
The presence of specific carbohydrates on the cell surface of particular types of cells opens up the possibility of creating vaccines. A hexasaccharide construct partly assembled by automated synthesis was shown to be immunogenic and to provide significant protection against malarial pathogenesis.
-
Special carbohydrate antigens, such as sialyl Lewis X and Globo-H, are known to be overexpressed on the surface of cancer cells. Patients immunized with synthetic carbohydrate-based vaccines produce antibodies that are reactive against malignant cells.
-
The presence of specific carbohydrates on the cell surface of particular cell populations presents the possibility of detecting these cells on the basis of their carbohydrate coat. This property is used in detecting even a small amount of bacteria with carbohydrate-functionalized fluorescent polymers.
Abstract
Carbohydrates present both potential and problems — their biological relevance has been recognized, but problems in procuring sugars rendered them a difficult class of compounds to handle in drug discovery efforts. The development of the first automated solid-phase oligosaccharide synthesizer and other methods to assemble defined oligosaccharides rapidly has fundamentally altered this situation. This review describes how quick access to oligosaccharides has not only contributed to biological, biochemical and biophysical investigations, but also to drug discovery. Particular focus will be placed on the development of carbohydrate-based vaccines, defined heparin oligosaccharides and aminoglycosides that have recently begun to affect drug discovery.
Similar content being viewed by others
Main
Nucleic acids, proteins and glycoconjugates, the three major classes of repeating biopolymers, are key to the signal transduction processes in living organisms. Both GENOMICS AND PROTEOMICS have evolved as very active areas in drug discovery. Many tools are available for determining the structure and function of nucleic acids and proteins, as well as their interactions. Fundamental breakthroughs in basic science and emerging new technologies have been the platform for advances toward a host of new therapeutics that involve nucleic acids and proteins. Protein–protein, protein–nucleic acid and nucleic acid–nucleic acid interactions are now targets of therapeutic approaches aiming to modify, enhance or disrupt these events. The third major class of biopolymers — carbohydrates and glycoconjugates — have seen less interest from a drug development perspective. The lack of carbohydrate-based drugs is not surprising considering that our understanding of fundamental glycobiology is a rather recent development. However, carbohydrates are an essential part of every cell surface and are crucial in cell surface recognition and information transfer. In many cases, oligosaccharides are attached to proteins or lipids to form glycoproteins or glycolipids that comprise part of the lipid bilayer (Fig. 1).
Two major technological breakthroughs have catapulted peptide- and oligonucleotide-based drug discovery forward: oligonucleotide and protein sequencing, which are now automated and allow for the composition of an unknown oligomer to be determined quickly and reliably1. Structure–activity relationships can be established rapidly and provide a starting point for structure–function studies and the design of modified oligomers. The synthesis of defined oligonucleotides2,3 and peptides4 can now be achieved by non-experts in an automated fashion. Modified oligonucleotides, peptides and proteins have found uses as both research tools and therapeutic agents.
Unlike oligonucleotides and peptides, carbohydrates are not just linear oligomers, but are often branched. The nine common monosaccharides found in mammalian cells can be combined in a dazzling variety of ways to form structures more diverse than those accessible with the twenty naturally occurring amino acids or the four nucleotides. This structural complexity renders the isolation of pure carbohydrates from natural sources extremely difficult, even when it is in principle possible. No amplification methods analogous to the POLYMERASE CHAIN REACTION (PCR) for DNA are available for carbohydrates. Until recently the identification of specific carbohydrate sequences responsible for a particular interaction had been hampered greatly by the unavailability of generally applicable sequencing methods. Although this shortcoming has been addressed by several groups, and sequencing techniques have been much improved, it remains a skill practiced by relatively few expert laboratories5.
Particularly in the initial stages of training a sequencing method, access to pure biopolymers becomes important. Sufficient quantities of pure material is key for biological, biochemical and biophysical studies and relies on chemical or enzymatic synthesis. Given the structural complexity of carbohydrates, regio- and stereoselectivity of glycosylation reactions is the key challenge for the assembly of oligosaccharides. Synthetic chemists have developed increasingly powerful and versatile methods that have resulted in the assembly of ever-more complex oligosaccharides and glycosaminoglycans. Still, the preparation of such structures remained technically difficult, extremely time consuming and has been carried out by a few highly specialized cognescenti. Advances in enzymatic synthesis6 have addressed some aspects of this challenge. Most importantly, the development of an automated synthesizer7,8 has fuelled the growing need for defined oligosaccharide structures as glycomics efforts gather momentum. Now automated oligosaccharide synthesis is beginning to accelerate drug discovery.
Synthesis of carbohydrates
Carbohydrates are unlike the other major classes of biopolymers often characterized by highly branched motifs. Each five- or six-membered ring monosaccharide unit has multiple sites of attachment to the next sugar moiety. Additionally, each glycosidic linkage connecting two sugar units can take on one of two possible isomeric forms. There are more than 1,000 different trisaccharides possible when the nine mammalian monosaccharides are combined9. The synthesis of oligosaccharides has been pursued for more than 100 years and the key coupling reaction, the glycosylation reaction10, is one of the most thoroughly studied transformations in organic chemistry (Box 1).
One alternative to traditional chemical synthesis for the procurement of carbohydrates is enzymatic methods. Enzymatic techniques rely on the high specificity prevalent in glycosyl-transferase-mediated bond formation11. Utilizing nucleotide diphosphosugars (NDPs) as building blocks, glycosyl transferases assemble complex carbohydrates in aqueous media. A major advantage of this method is the ability to prepare sophisticated structures without the need for PROTECTING GROUP manipulations on either the building blocks or the desired product. Certain carbohydrates can be prepared using a particular transferase, but the narrow scope of transferase-mediated glycosylations necessitates the isolation and purification of multiple enzymes to synthesize diverse structures. Additionally, the high cost associated with nucleotide diphospho-sugars necessitates the regeneration of NDPs12,13 in situ via immobilized enzymes for large-scale synthesis14. The field of enzymatic reglycosylation has made a major impact in the area of therapeutic glycoproteins15. Considerable research in this area is currently ongoing and should lead to new, more efficient and flexible enzymatic methods16.
Orthogonal one-pot methods
The desire to streamline the synthesis of carbohydrates has led to the design and evaluation of efficient ONE-POT METHODS. An chemoselective solution-phase method that relies on thioglycosides as glycosylating agents is the OptiMer strategy of Wong17. Analysis of the reactivity profiles for more than 100 different thioglycosides using a computer program enables the prediction of the optimal set of building blocks required to generate a given oligosaccharide. This method involves the one-pot sequential addition of building blocks with decreasing reactivity18. The reactions are performed manually in solution such that the oligosaccharide chain is extended from the non-reducing to the reducing end. Factors affecting the relative reactivity value (RRV) for a thioglycoside include the type of sugar and the nature of the protecting groups used.
This approach is useful for the synthesis of shorter oligosaccharide sequences, as exemplified by the synthesis of the Globo-H hexasaccharide19,20,21. Currently, efforts are underway to develop a set of building blocks from which the majority of carbohydrate sequences can be prepared. A challenge inherent to this method is evident when structures containing more than one identical linkage are desired. The need for orthogonal reactivity and sequential addition of donors necessitate the use of more than one building block (with different RRVs) for the same linkage, thereby adding to the overall number of building blocks needed. Although there are still limitations to the OptiMer method, it represents the first example of a computer-assisted program for solution-phase oligosaccharide synthesis.
Another notable example of orthogonal one-pot glycosylations using thioglycosides is that of Takahashi and co-workers22. A library of 54 linear trisaccharides was prepared in parallel on a commercial synthesizer using the strategy outlined above. Similarly, 18 branched trisaccharides were also assembled in excellent overall yield (64–99%). This highly efficient procedure and the successful use of a manual synthesizer should lead to more advances in instrumentation. Restrictions on the overall length of the oligosaccharides prepared by one-pot methods currently hinder a more widespread use of this technique.
Automated solid-phase assembly
Solution-phase oligosaccharide synthesis remains a slow process because of the need for iterative coupling and deprotection steps, with purification at each step along the way. Solid-phase synthesis has proven extremely efficient for the assembly of peptides and oligonucleotides as it does not require purification after each reaction step, utilizes excess reagent to drive reactions to completion and lends itself to automation. To alleviate the need for repetitive purification events required during solution-phase oligosaccharide assembly, SOLID-PHASE techniques have been developed23,24.
A series of different approaches to solid-phase oligosaccharide synthesis25 were described and all critical aspects including the choice of synthetic strategy, differentially protected glycosylating agents, solid support materials and linkers to attach the first monosaccharide to the support matrix were explored. The first automated solid-phase oligosaccharide synthesizer, based on a modified peptide synthesizer, was introduced in 2001 (Fig. 2)8. Attachment of the reducing end sugar to the solid support at the anomeric position allows for the step-wise incorporation of one mono- or disaccharide building block at a time. The use of UV-active protecting groups enables real-time monitoring of the success of the automated synthesis, as is common for the automated synthesis of peptides and oligonucleotides. Glycosyl phosphates26 and glycosyl trichloroacetimidates27 have proven to be extremely powerful building blocks for automated assembly that also draws on novel linkers to connect the first sugar to the solid support. The first automated synthesizer was based on a modified peptide synthesizer, and introduced several innovative solutions to the protecting group, the building block, the linker and the analysis challenge. Utilizing this automated synthesizer, a host of biologically important oligosaccharides was prepared to demonstrate the power of this approach. The synthesis of complex carbohydrates, such as the Lewis X-Lewis Y (Lex-Ley) nonasaccharide ANTIGEN found on tumour cells, was accomplished in less than 1 day28. This process required well over 1 year when using the most sophisticated solution-phase synthesis methods.
A retrosynthesis of the fully protected Lex-Ley nonasaccharide 1 is shown in Fig. 3a. With a sequential strategy that used five different glycosyl phosphates (2–6) as building blocks and starting with the functionalized resin 7, an automated solid-phase synthesis of this biologically important compound was possible28. Coupling of the glycosyl phosphate monomers 2–6 was carried out at −15 °C. Removal of the 9-fluorenylmethoxycarbonyl (Fmoc) and the levulinoyl (Lev) groups was achieved at room temperature. The coupling as well as the deprotection steps were repeated at least twice to ensure consistently high yields. A general cycle for the installation of one building block is shown in Fig. 3b. Table 1 gives an overview of the time required for each step. Repetition of these cycles with the corresponding building blocks completed the assembly of the nonasaccharide in less than 1 day. Cleavage of the ester linker from the resin over a period of 6 hours provided the crude oligosaccharides before high-pressure liquid chromatography purification produced the fully protected Lex-Ley nonasaccharide in 6.5% yield28.
a | Retrosynthesis based on five different building blocks 2–6 and the resin with a hydroxyl group 7. b | Automated oligosaccharide synthesis with glycosyl phosphates. Initial glycosylation of resin-bound acceptor 7 produces a coupling product that is subsequently deprotected. Iteration of coupling and deprotection cycles with monosaccharide building blocks 2–6 followed by cleavage of the resin-bound oligosaccharide and purification furnishes nonasaccharide 1.
The currently available automated synthesizer has accelerated access to many carbohydrates several hundredfold, still some sequences remain difficult to make and not all oligosaccharides can be assembled on a solid support. The most time-consuming step for the procurement of pure carbohydrates is the synthesis of sufficient quantities of building blocks. In the near future those building blocks will become commercially available, which will greatly facilitate synthetic efforts. Improvements including new building blocks and methodologies to access all possible linkages, accelerated protocols for the deprotection of synthetic oligosaccharides and better robotic systems that will allow for multiple carbohydrates to be synthesized in parallel are under development.
Screening carbohydrate interactions
Protein–protein, nucleic acid–protein and nucleic acid–nucleic acid interactions have been the focus of drug development for several years. The search for small molecules that can efficiently and specifically disrupt a particular protein–protein interaction has been on for several years now, and several such compounds have reached clinical trials29. The interaction of transcription factors with DNA strands has also been studied in detail to try to achieve transcriptional regulation utilizing non-natural molecular entities30. Nucleic acid–nucleic acid interactions have been used in antisense and RNA interference approaches to drug discovery31. Glycoconjugates naturally decorate the surface of mammalian cells and are involved in cell–cell interactions32 based on carbohydrate–protein or carbohydrate–carbohydrate binding. In addition, carbohydrates have been found to specifically interact with bacterial RNA as is the basis for the mode of action of aminoglycoside antibiotics.
Carbohydrate–protein interactions. The realization that SELECTINS (E-, L- and P-selectins) bind to carbohydrates to initiate the rolling of leukocytes at the onset of the inflammatory cascade33 sparked efforts to create small-molecule inhibitors of these interactions. The relatively weak binding interactions (millimolar KD) and multivalent mode of binding made the development of antagonists very difficult and eventually doomed the programmes at several companies — thereby earning carbohydrates the reputation of being undruggable. However, bearing in mind that the glycosaminoglycans class of carbohydrates — for example, heparin — interact with certain receptors with a nanomolar binding interaction, screening of carbohydrate–protein interactions could indeed yield interesting targets.
High-throughput screening methods34,35,36 for rapidly identifying carbohydrate–protein interactions are hindered by limited access to pure materials. Procurement of synthetically defined sugars has accelerated the development and use of carbohydrate arrays and their use to screen for interesting interactions. Applications are manifold: the arrays can be used to discover novel carbohydrate–protein interactions, and to define the EPITOPES recognized by disease-related or vaccine-induced antibodies. The chips also allow for the definition of carbohydrate antigens for vaccine development.
Carbohydrate–RNA interactions. Aminoglycosides are carbohydrate antibiotics that contain amino sugars and are composed of two to five monomers (Fig. 4). Clinically, these compounds are used as broad-spectrum antibiotics against various important bacteria. Aminoglycosides exert their antibacterial effect by binding to bacterial ribosomes and inhibiting protein synthesis. The most common binding site for this class of drugs is the A-site in the small ribosomal subunit, or 30S portion, of the bacterial ribosome. Therapeutic efficacy of aminoglycosides has decreased recently due to increased antibiotic resistance37. Resistance to aminoglycosides can be either acquired through the transfer of plasmid DNA or from overexpression of endogenous enzymes. Several mechanisms cause resistance, including decreased uptake into cells, mutation of the target, binding to proteins and covalent modification of the drug by enzymes. Enzymatic modification, and consequently a large decrease in binding affinity to the therapeutic target, is the most common resistance mechanism against aminoglycosides38. To combat the growing threat drug-resistant bacteria pose to human health, new antibiotics must be identified. The discovery of such compounds benefits from high-throughput methods using the micorarray technique39,40 to identify compounds that weakly bind to resistance- and toxicity-causing proteins and strongly bind to therapeutic targets.
Aminoglycoside MICROARRAYS were constructed by nonspecific immobilization of the antibiotics onto glass slides using a DNA arraying robot to provide a versatile platform for probing the interactions with various targets. Arrays were probed with RNA mimics of the bacterial and human A-sites to establish that the microarray method can serve as a screen for RNA binding and specificity. Arrays were also incubated with two acetyltransferase (AAC)-resistance enzymes, an AAC(6′) from Salmonella enterica and an AAC(2′) from Micobacterium tuberculosis. Binding of these enzymes to the aminoglycosides correlated well with an earlier calorimetric study of binding affinity39.
Oligosaccharides as drugs
Some highly successful drugs have been based on carbohydrates despite the lack of tools to examine glycobiology in the way genomics and proteomics have been approached. In addition to monosaccharide-derived drugs (Table 2) two oligosaccharide drugs, acarbose and heparin, have been highly successful. The advent of carbohydrate sequencing and synthesis now makes it possible to greatly accelerate the development of defined drugs and oligosaccharide-based vaccines beyond the examples described here.
Acarbose. With an increasing percentage of the world's population overweight, metabolic diseases such as diabetes mellitus are becoming an ever-more serious health problem and have created a rapidly increasing market for drugs to treat this condition. For a long time the basic treatment of diabetes mellitus was defined by strict dietary control. Reduced intake of carbohydrates and the intake of many small portions in place of large meals can prevent excessive blood glucose levels, excessive blood insulin levels and increased triglyceride blood levels. Often this regimen is not sufficient to control the disease and intervention with drugs is required. Carbohydrates are a principal component of human food, and starch and sucrose are the main components (80–90%). Di- and polysaccharides must be enzymatically split in the intestinal tract before they can be utilized by the organism. Interference with the intestinal carbohydrate digestion by acarbose (Precose; Bayer AG)41, an α-GLUCOSIDASE and α-amylase inhibitor, is one way to regulate and retard carbohydrate digestion, control the rate of absorption of monosaccharides and thereby influence the intermediary metabolism of the carbohydrates. Acarbose is a pseudo-oligosaccharide of microbial origin and has become a blockbuster diabetes drug. Acarbose is produced by fermentation but demonstrates that defined oligosaccharides or their close analogues can yield active drugs in areas of great need and in lucrative markets. Acarbose can therefore be viewed as an early carbohydrate drug that did not require sequencing or synthesis as it was obtained by screening.
Heparin. An older (and even better selling) carbohydrate-based drug is heparin (Box 2). This sulphated glycosaminoglycan is isolated from animal organs and has been used clinically as an antithrombotic agent since the 1940s. Heparin activates antithrombin III (AT-III), a serine protease inhibitor that blocks thrombin and factor Xa in the coagulation cascade42. It binds to AT-III very specifically and with high affinity (nanomolar range). However, the composition of this drug, consisting of a highly heterogeneous mixture of polysaccharides, is ill defined. Severe side effects, including heparin-induced thrombocytopaenia (HIT), bleeding and allergic reactions were observed43. If it were to be discovered today, heparin would never reach the market.
In the early 1980s, heparin was submitted to chemical or enzymatic fragmentation, and the resulting low-molecular-weight heparins (LMWHs) became the market leaders in antithrombotics, with current world sales of about US$2 billion per year. Advantages of LMWH over full-length heparin include greater bioavailability, longer half-life, no need for constant patient monitoring, more predictable anticoagulant activity and fewer side effects. Despite these advantages, LMWHs are still heterogeneous and are less active against thrombin inhibition than heparin. Furthermore, LMWHs are not free of adverse effects: they can cause major haemorrhagic events, osteoporosis, HIT, skin necrosis, allergic skin reactions and elevations in the level of liver enzymes.
A specific pentasaccharide (Glc6S,NS-GlcA-GlcNS,3S,6S-IdoA2S-GlcNS,6S) that is responsible for the anticoagulant property was also identified in the early 1980s44. Soon, two companies initiated programmes designed to create a synthetic heparin. The challenge was enormous — a multistep synthesis had to be devised to prepare one of the most complex carbohydrates made at that time at scale for clinical trials and at competitive cost. The synthetic programme established a structure–function relationship of the pentasaccharide and explored a series of close analogues with the goal of finding a more potent molecule that could be synthesized more readily. Following a development period of well over 10 years, Sanofi and Organon created a synthetic analogue of this pentasaccharide named Fondaparinux. This compound has been marketed as Arixtra since 2002, with a market value of US$1 billion per year45. Overcoming the pessimistic view of 15 years ago, it has been shown that the multi-kilogram synthesis of the highly pure pentasaccharide can be performed successfully in industry. The half-life of arixtra in humans is approximately 17 hours, allowing for once-per-day administration, instead of two or three injections of heparin. The use of the defined pentasaccharide in major orthopaedic surgery led to a decrease in the risk of thrombosis of more than 50% relative to LMWH46.
Carbohydrates as vaccines
The presence of specific carbohydrates on the cell surface of particular types of cells presents the option of creating vaccines. Parasites carry sugars that are distinct from those of their hosts. In contrast to proteins that can be changed by the parasites without major genetic upheaval, carbohydrates are more evolutionarily stable. An immune response against the carbohydrate antigens that results in the killing of the target cells is the basis for vaccine development. Vaccines based on carbohydrate antigens have been used widely for several decades against a host of diseases47. Initially, only vaccines against bacterial infections were produced using carbohydrate antigens that were isolated from biological sources. In the past few years, intense efforts focused on the use of completely defined, synthetic carbohydrate antigens. In addition to carbohydrate-based vaccines for bacterial infections (such as menengitis), viral infections (including HIV) and parasitic infections (such as malaria and leishmaniasis), carbohydrate-based cancer vaccines have also been explored.
Bacterial infections
The surface of the cell of many bacterial species is covered by polysaccharides that occur in the form of capsules, glycoproteins or glycolipids. In Gram-negative bacteria, the lipopolysaccharide (LPS) covers about 40% of the bacterial surface. Capsular polysaccharides (CPS) are either homopolymers or are made of repeating units normally consisting of two to six sugar residues.
Studies into its immunoprotective effects revealed that CPS elicit type-specific protective immune responses in adults, but infants and young children do not respond with type-specific antibodies. Such antibodies confer protection, and vaccination with polysaccharides reduces the carrier rate of bacteria. Neoglycoconjugates consisting of oligosaccharides covalently linked to a carrier protein induced high titres of protective antibodies. Because of the introduction of antibiotics, there seemed to be no need for antibacterial vaccine development.
As antibiotic resistance began to evolve ever-more rapidly in the 1970s, the development of antibacterial carbohydrate vaccines was pursued, building on fundamental discoveries in immunology. Improved analytical tools enabled the identification of the exact chemical structure48,49 of carbohydrate antigens and the development of new, polysaccharide-based vaccines. Several vaccines based on purified CPS or on neoglycoconjugates are now commercially available, such as vaccines against Neisseria meningitidis, Steptococcus pneumoniae, Haemophilus influenza type b (Hib) and Salmonella typhi50. For instance, meningitis and pneumonia caused by Hib have been essentially eradicated where national vaccination programmes with protein conjugate vaccines have been implemented51.
Despite the significant advances in the development of carbohydrate-based vaccines several challenges remain to be tackled. First, carbohydrate antigens have a large degree of antigenic variation as evidenced by the structural differences in the surface polysaccharides within the same species. Second, homology between carbohydrate structures present on bacterial surfaces and on host cell membranes have been reported. And last, polysaccharide antigens are mostly poor immunogens due to their T-lymphocyte-independent nature. Often, the anti-polysaccharide immune response is characterized by a lack of T-lymphocyte memory. Children below 2 years of age and the elderly respond poorly to polysaccharide antigens. Conjugate vaccines consisting of a carbohydrate antigen and an immunogenic protein can overcome this immunogenicity problem.
Parasitic infections
Malaria. Malaria is caused by single-celled parasites of the genus Plasmodium, of which Plasmodium falciparum is the most pathogenic. When an infected mosquito bites a human host, the parasite is transferred. After several stages, red blood cells are infected, causing red-cell lysis and the release of new parasites. This subsequently leads to chills and fever, which are common symptoms of this severe disease that infects 5–10% of humanity and kills more than 2 million people a year52. Current malaria treatments are often impractical, and drug resistance is a growing problem. At the same time, there is still no effective malaria vaccine.
Recently, it was shown that the malaria parasite P. falciparum expresses a large amount of glycosylphosphatidylinositol (GPI) on its cell surface53. The inflammatory cascade triggered by this parasite GPI is responsible for much of malaria's morbidity and mortality. The malarial GPI can activate macrophages and induce cascades resulting in the production of mediators such as nitrous oxide (NO) and tumour-necrosis factor-α (TNFα)51.
To unequivocally define this antigen and evaluate its potential use as a new vaccine, a synthetic version of the hexasaccharide malaria toxin (Fig. 5a)54 was reacted with a linker and conjugated to the maleimide-activated carrier protein KEYHOLE LIMPET HAEMOCYANIN (KLH). Mice treated with chemically synthesized GPI attached to this protein were substantially protected from death by malaria. Between 60 and 75% of the vaccinated mice survived, whereas the survival rate for unvaccinated mice was only 0–9%55. As a result of the differences in the GPI structures of humans and Plasmodium, antibodies to the synthetic GPI conjugate did not crossreact with human GPI. For future applications it should be noted that only miniscule amounts (10−9−10−7 g per person) of the hexasaccharide, which was partly assembled by automated synthesis, would be needed for vaccination. The preclinical model revealed that a non-toxic GPI oligosaccharide coupled to a carrier protein is immunogenic and provides significant protection against malarial pathogenesis56. Interestingly, the immunization of the mice did not alter the infection rates of the animals and overall parasitaemia, indicating that the antibody to the GPI neutralized toxicity without killing the parasites. An antitoxic antipathogenesis oligosaccharide vaccine against malaria is currently in advanced preclinical evaluation.
Leishmaniasis. Leishmaniasis, a tropical disease that afflicts more than 12 million people worldwide57, is spread by the bite of infected sandflies. Therapeutic approaches are difficult, because the protozoan parasite Leishmania resides in the macrophages, the very part of the immune system that is designed to destroy it. A potent vaccine that would facilitate destruction of the parasite on transfer from the sandfly into the human host would be beneficial. Any unique feature the parasite exposes on its cell surface could be used for the development of a vaccine. Lipophosphoglycans (LPGs)58 are ubiquitious on the cell surface of the parasites and are composed of a GPI anchor, a repeating phosphorylated disaccharide and different cap oligosaccharides (Fig. 5b). The phosphoglycan part is a disease-promoting antigen56, but its high molecular mass and heterogeneity preclude its use as a vaccine. Therefore, investigations focused on a structurally well-defined leishmaniasis carbohydrate vaccine, based on the cap tetrasaccharide59. The branched tetrasaccharide was assembled stepwise from monosaccharide building blocks by automated solid-phase synthesis60 and conjugated either to the carrier protein KLH or virosomal particles61. Initial results of the immunological evaluation in mice have been promising.
Cancer
Specific types of glycolipids or glycoproteins present on normal cells are more highly expressed in certain tumours. High levels of expression on tumour cells causes an antibody response, and renders these cell-surface glycoconjugates tumour-associated antigens — the basis for the development of antitumour vaccines62,63.
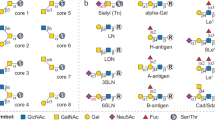
Carbohydrate antigens such as sialyl Lex and Globo-H (Fig. 6) are suitable targets for both active and passive anticancer immunotherapies because they have been carefully characterized as being overexpressed on the surface of malignant cells64. Globo-H is found on the cell surface of breast61, prostate and ovarian cancer cells65,66, whereas sialyl Lex is observed in various types of cancer. Suitable monoclonal antibodies have been obtained and serve as serological markers for immunological studies64. Patients were immunized with synthetic carbohydrate vaccines to produce antibodies reactive against cancer cells. The production of such antibodies should mitigate tumour spread. Other tumour-associated carbohydrate antigens suppress invasiveness and metastatic potential, such as blood group A antigen in primary lung carcinoma. Most biochemical mechanisms by which changes in glycosylation promote or suppress tumour metastasis and invasion are unknown. Sialyl Lex and Lea function as E-selectin epitopes that promote tumour-cell interactions with endothelial cells.
Encouraging preliminary results with anticancer vaccine candidates that use tumour-associated carbohydrate antigens indicative of enhanced malignancy have emerged after clinical trials. Examples include sialyl TN antigen for suppression of breast cancer, GM2 and GD3 for melanoma, and Globo-H in the case of prostate and breast cancer. Vaccine development can be extended to other tumour-associated carbohydrate antigens using the following criteria: the antigen is expressed highly on tumour cells; high antibody production depending on the clustering of antigen used in vaccine and/or choice of appropriate carrier protein or lipid; high T-cell response; and expression of the same antigen in normal epithelial tissues is unlikely to pose a major obstacle. Idiotypic anticarbohydrate antibodies that mimic the surface profile of carbohydrate antigens, when administered to patients, elicit an anticarbohydrate antibody response, thereby providing an effect similar to that of tumour-associated carbohydrate antigens for suppression of tumour progression.
There are grounds for both optimism and caution in using cancer vaccines based on tumour-associated carbohydrate antigens. The immune response against carbohydrate antigens predominantly induces a B-cell rather than a T-cell response. Antibodies could provide a means of eradicating tumour cells circulating in the blood stream and micrometastases, thereby providing protection from tumour reoccurrence. Administration of monoclonal antibodies against carbohydrate antigens in mouse models has progressed to the point where micrometastases can be eliminated. Cancer patients in clinical studies have also responded favourably to passively administered antibodies, resulting in prolonged disease-free survival and prognosis. Aggressive local therapies followed by vaccination hold the potential to result in long-term control, even over metastatic cancers.
There is another potential complication in the use of tumour-associated carbohydrate antigens in cancer vaccines. Cancer and normal cells growing in tissue culture generally show minimal levels of expression of such antigens. The immense difficulties associated with their purification from such sources render them virtually non-available as homogeneous starting materials for a clinical programme. Accordingly, organic chemists have a key role in the development of such cancer vaccines. Synthetic chemists have to procure pure antigens in sufficient quantities to advance a clinical programme. Conjugation of the synthetic antigen to form a vaccine is again handled by chemists. Most tumour antigens, including carbohydrate tumour antigens, are generally poor immunogens and require an appropriate immunogenic carrier to achieve an appropriate response. Therefore, an additional challenge to cancer vaccine strategies is the successful delivery of the synthetic tumour antigens in a favourable molecular context for eliciting a therapeutically useful immunological response.
Use of carbohydrates in diagnostics
The presence of specific carbohydrates on the cell surface of particular cell populations presents the possibility to detect these cells on the basis of their carbohydrate coat. Diagnostic tools that recognize specific carbohydrates on the surface of certain cell types will be important for the detection of cancers.
Carbohydrate arrays. Carbohydrate microarrays can be used to investigate the carbohydrate-binding specificity of intact bacterial cells. The carbohydrate-coated array surface is ideal for measurements of binding specificities because the surface presents carbohydrate ligands in a manner that facilitates multivalent binding67. Cell adhesion on the arrays can be readily visualized using cell-permeable fluorescent dyes to stain the cells' nucleic acids. The array-based method enables assay miniaturization and requires only minimal amounts of ligand and cells when compared with solution measurements or experiments in 96-well plates. Carbohydrate–cell interactions can be detected in homogeneous and heterogeneous solutions that contain bacteria. Reliable detection even in complex mixtures that mimic body fluids illustrates the potential this method could hold in the future as a pathogen-specific diagnostic test.
Carbohydrate-functionalized fluorescent polymers. In many cases, bacteria as well as viruses bind to carbohydrates displayed on the host cells they infect. Escherichia coli binds mannose, and influenza virus binds to sialic acid, to name two examples68,69. To ensure the high binding affinity necessary for strong adhesion and successful infection of the cell, the pathogen often uses multivalent interactions (Fig. 7a)70. Conducting polymers displaying carbohydrates can simulate these binding events and serve as ideal material for the detection of even small amounts of pathogen.
Recently, a carbohydrate-functionalized polymer (Fig. 7b) that can be used for the detection of E. coli by multivalent interactions was reported71. Experiments with two bacterial strains differing in their mannose-binding properties revealed that the mannose-functionalized polymer imparted strong fluorescence to mannose-binding E. coli. Even separation and rinsing procedures are not able to remove the bacteria from the polymer. By contrast, the mutated strain unable to bind mannose showed no signal and no aggregation of bacteria. The binding events involving the functionalized polymers and the bacteria were followed under the microscope (Fig. 8). Mutant bacteria that lost the ability to bind to mannose did not aggregate to the polymer whereas the mannose-binding bacteria formed fluorescent clusters with cell numbers from tens to several thousand cells. The detection limit of this method is in the range of 103−104 bacteria. However, many pathogens bind the same carbohydrates — for example, E. coli as well as Salmonella enterica bind to mannose. Selectivity can be improved using multiple carbohydrates on arrays72.
a | Mutant Escherichia coli that does not bind to carbohydrate-functionalized polymer. b | A fluorescent bacterial aggregate forms due to multivalent interactions between the mannose-binding bacterial pili and the polymer (super- imposed fluorescence and transmitted light images). c | Fluorescence microscopy image of a large fluorescent bacterial cluster. d | Conventional fluorescence spectra of polymer (green) and normalized fluorescence spectra of a bacterial cluster obtained using confocal microscopy (orange).
Conclusions and perspectives
For decades carbohydrates were only studied with respect to their role in energy storage and supply. Biosynthesis and biodegradation pathways were elucidated; the function of oligosaccharides in biologically important recognition processes became evident much later. First, problems with the isolation, purification and structure determination of miniscule amounts of carbohydrate had to be overcome. Second, powerful chemical and enzymatic methods for the synthesis of oligosaccharides have been established. Glycosyl phosphates and glycosyl trichloroacetimidates have been used as extremely efficient building blocks in a fully automated oligosaccharide synthesizer, dramatically reducing the time needed for synthesis. Suitable protection and deprotection strategies led to the assembly of linear and even branched structures in a fully automated fashion.
Carbohydrate-based drugs have been used since the discovery of heparin in the 1940s. Elaborate synthetic strategies, such as different modular approaches, have paved the way to access almost all possible heparin structures. The presence of specific carbohydrates on the cell surface of particular types of cells opens the option of creating vaccines. Particular success has been achieved in the case of malaria. Tumour-associated antigens such as Globo-H constructs have also shown promising results.
Carbohydrate-based vaccines are now beyond the proof-of-principle stage. Rapid access to a plethora of different oligosaccharides through automated synthesis will enable future glycobiologists to screen large numbers of carbohydrates that are thought to have previously unimagined roles in biological systems. The knowledge gained from glycomics efforts will complete our understanding of all kinds of biochemical processes and provide access to a new arsenal of weapons for the fight against severe diseases. Future research will answer structural and mechanistic questions with regard to the immunological response in more detail. Carbohydrate-binding proteins, responsible for the attachment of viruses and parasites, will be identified and will provide further insights at the molecular level in various diseases. We are still just beginning to understand the importance of sugars in our lives beyond pasta, cake and chocolate. There is mounting evidence that the future of medicine will be a sweet one. Future therapeutic or preventive battles in our bodies will be fought or at least supported by synthetically obtained sugars.
References
Hunkapiller, T., Kaiser, R. J., Koop, B. F. & Hood, L. Large-scale and automated DNA sequence determination. Science 354, 59–67 (1991).
Caruthers, M. H. Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 (1985). The essentials for the synthetic assembly of genes are presented.
Caruthers, M. H. Chemical synthesis of DNA and DNA analogs. Acc. Chem. Res. 24, 278–284 (1991).
Atherton, E. & Sheppard, R. C. Solid-Phase Peptide Synthesis: A Practical Approach (Oxford Univ. Press, Oxford, 1989).
Sears, P. & Wong, C. -H. Toward automated synthesis of oligosaccharides and glycoproteins. Science 291, 2344–2350 (2001).
Wong, C. -H. Enzymic and chemo-enzymic syntheses of carbohydrates. Pure Appl. Chem. 67, 1609–1616 (1995).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001). The first automated solid-phase oligosaccharide synthesizer is presented. Linear and branched structures up to dodecasaccharides are prepared.
Seeberger, P. H. Automated carbohydrate synthesis to drive chemical glycomics. Chem. Commun. 1115–1121 (2003).
Laine, R. A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the isomer barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767 (1994).
Schmidt, R. R., Castro-Palomino, J. C. & Retz, O. New aspects of glycoside bond formation. Pure Appl. Chem. 71, 729–744 (1999).
Koeller, K. & Wong, C. -H. Synthesis of complex carbohydrates and glycoconjugates: enzyme-based and programmable one-pot strategies. Chem. Rev. 100, 4465–4493 (2000).
Wong, C. -H., Halcomb, R. L., Ichikawa, Y. & Kajimoto, T. Enzymes in organic synthesis: application to the problems of carbohydrate recognition. Part 1. Angew. Chem. Int. Ed. Engl. 34, 412–432 (1995).
Wong, C. -H., Halcomb, R. L., Ichikawa, Y. & Kajimoto, T. Enzymes in organic synthesis: application to the problems of carbohydrate recognition. Part 2. Angew. Chem. Int. Ed. Engl. 34, 521–546 (1995).
Chen, X. et al. Sugar nucleotide regeneration beads (superbeads): a versatile tool for the practical synthesis of oligosaccharides. J. Am. Chem. Soc. 123, 2081–2082 (2001).
Delorme, E. et al. Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31, 9871–9876 (1992).
Jiang, J., Biggins, J. B. & Thorson, J. S. A general enzymatic method for the synthesis of natural and 'unnatural' UDP- and TDP-nucleotide sugars. J. Am. Chem. Soc. 122, 6803–6804 (2000).
Ye, X. -S. & Wong, C. -H. Anomeric reactivity-based one-pot oligosaccharide synthesis: a rapid route to oligosaccharide libraries. J. Org. Chem. 65, 2410–2431 (2000).
Douglas, N. L., Ley, S. V., Lucking, U. & Warriner, S. L. Tuning glycoside reactivity: new tool for efficient oligosaccharide synthesis. J. Chem. Soc., Perkin Trans. 1 51–65 (1998).
Lassaletta, J. & Schmidt, R. R. Glycosyl imidates. Part 75. Synthesis of the hexasaccharide moiety of globo H (human breast cancer) antigen. Liebigs Ann. 1417–1423 (1996). The first total synthesis of Globo-H hexasaccharide is reported.
Park, T. K. et al. Total synthesis and proof of structure of a human breast tumor (Globo-H) antigen. J. Am. Chem. Soc. 118, 11488–11500 (1996).
Bosse, F., Marcaurelle, L. A. & Seeberger, P. H. Linear synthesis of tumor-associated carbohydrate antigens Globo-H, SSEA-3, and Gb3. J. Org. Chem. 67, 6659–6670 (2002).
Takahashi, T., Adachi, M., Matsuda, A. & Doi, T. Combinatorial synthesis of trisaccharides via solution-phase one-pot glycosylation. Tetrahedron Lett. 41, 2599–2603 (2000).
Frechet, J. M. in Polymer-supported Reactions in Organic Synthesis (eds Hodge, P. & Sherrington, D. C.) 407–434 (Wiley, Chichester, 1980). Provides a review of solid-phase oligosaccharide synthesis before 1980.
Seeberger, P. H. & Haase, W. -C. Solid-phase oligosaccharide synthesis and combinatorial carbohydrate libraries. Chem. Rev. 100, 4349–4393 (2000). A recent review of solid-phase oligosaccharide assembly.
Seeberger, P. H. (ed.) Solid Support Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries (Wiley-Interscience, Chichester, 2001).
Plante, O. J., Andrade, R. B. & Seeberger, P. H. Synthesis and use of glycosyl phosphates as glycosyl donors. Org. Lett. 1, 211–214 (1999).
Schmidt, R. R. & Kinzy, W. Anomeric-oxygen activation for glycoside synthesis: the trichloroacetimidate method. Adv. Carbohydr. Chem. Biochem. 50, 21–123 (1994).
Love, K. R. & Seeberger, P. H. Automated solid-phase synthesis of protected tumor-associated antigen and blood group determinant oligosaccharides. Angew. Chem. Int. Ed. Engl. 43, 602–605 (2004). The synthesis of Lewis oligosaccharides was successfully performed on the automated oligosaccharide synthesizer starting from five different monomeric building blocks.
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nature Rev. Drug Discov. 1, 493–502 (2002).
Manson, M. M. et al. Modulation of signal-transduction pathways by chemopreventive agents. Biochem. Soc. Trans. 28, 7–12 (2000).
Wilson, W. D., Ratmeyer, L., Zhao, M., Strekowski, L. & Boykin, D. The search for structure-specific nucleic acid-interactive drugs: effects of compound structure on RNA versus DNA interaction strength. Biochemistry 32, 4098–4104 (1993).
Geijtenbeek, T. B. H. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune response. Cell 100, 575–585 (2000).
Kansas, G. S. Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287 (1996).
Barnes-Seemann, D., Park, S. B., Koehler, A. N. & Schreiber, S. L. Expanding the functional group compatibility of small molecule microarrays: discovery of novel calmodulin ligands. Angew. Chem. Int. Ed. Engl. 42, 2478–2481 (2003).
Fukui, S., Feizi, T., Galustian, C., Lawson, A. M. & Chai, W. Oligosaccharide microarrays for high throughput detection and specificity assignments of carbohydrate-protein interactions. Nature Biotechnol. 20, 1011–1017 (2002).
Hergenrother, P. J., Depew, K. M. & Schreiber, S. L. Small molecule microarrays: covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 122, 7849–7850 (2000).
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000).
Llano-Sotelo, B., Azucena, E. F. Jr., Kotra, L. P., Mobashery, S. & Chow, C. S. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem. Biol. 9, 455–463 (2002).
Disney, M. D., Magnet, S., Blanchard, J. S. & Seeberger, P. H. Aminoglycoside microarrays to study antibiotic resistance. Angew. Chem. Int. Ed. Engl. 43, 1591–1594 (2004).
Disney, M. D. & Seeberger, P. H. Aminoglycoside microarrays to explore interactions of antibiotics with RNAs and proteins. Chem. Eur. J. 10, 3308–3314 (2004).
Truscheit, E. et al. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew. Chem. Int. Ed. Engl. 20, 744–761 (1981).
Capila, I. & Linhardt, R. J. Heparin–protein interactions. Angew. Chem. Int. Ed. Engl. 41, 390–412 (2002).
Tabeur, C. et al. Oligosaccharides corresponding to the regular sequence of heparin: chemical synthesis and interaction with FGF-2. Bioorg. Med. Chem. 7, 2003–2012 (1999).
Petitou, M., Casu, B. & Lindahl, U. 1976–1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 85, 83–89 (2003).
Petitou, M. & van Boeckel, C. A. A. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. Engl. 43, 3118–3133 (2004). An excellent account of the state-of-the-art in heparin drug research.
Lee, J. -C., Lu, X. -A., Kulkarni, S. S., Wen, Y. -S. & Hung, S. -C. Synthesis of heparin oligosaccharides. J. Am. Chem. Soc. 126, 476–477 (2004).
Goldblatt, D. Recent developments in bacterial conjugate vaccines. J. Med. Microbiol. 47, 563–567 (1998).
Talbo, G. & Mann, M. Aspects of the sequencing of carbohydrates and oligonucleotides by matrix-assisted laser desorption/ionization post-source decay. Rap. Commun. Mass Spectr. 10, 100–103 (1996).
De Waard, P., Boelens, R., Vuister, G. W. & Vliegenthart, J. F. G. Structural studies by proton/carbon-13 two-dimensional and three-dimensional HMQC-NOE at natural abundance on complex carbohydrates. J. Am. Chem. Soc. 112, 3232–3234 (1990).
Sood, R. K., Fattom, A., Pavliak, V. & Naso, R. B. Capsular polysaccharide–protein conjugate vaccines. Drug Discov. Today 1, 381–387 (1996).
Tachado, S. D. et al. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. PNAS 94, 4022–4027 (1997).
World Health Organization World Malaria situation 1990. World Health Stat. Q. 45, 257–266 (1992).
Berhe, S., Schofield, L., Schwarz, R. T. & Gerold, P. Conservation of structure among glycosylphosphatidylinositol toxins from different geographic isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 103, 273–278 (1999).
Hewitt, M. C., Snyder, D. A. & Seeberger, P. H. Rapid synthesis of a glycosylphosphatidylinositol-based malaria vaccine using automated solid-phase oligosaccharide synthesis. J. Am. Chem. Soc. 124, 13434–13436 (2002).
Schofield, L., Hewitt, M. C., Evans, K., Siomos, M. -A. & Seeberger, P. H. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418, 785–789 (2002). This study identified a novel malaria vaccine candidate.
Mitchell, G. F. & Handman, E. The glycoconjugate derived from a Leishmania lipopolysaccharide confers parasite survival in macrophages. Parasite Immun. 8, 255–263 (1986).
Herwaldt, B. L. Leishmaniasis. Lancet 354, 1191–1199 (1999).
Turco, S. J. & Descoteaux, A. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46, 65–94 (1992).
Hewitt, M. C. & Seeberger, P. H. Solution and solid-support synthesis of a potential leishmaniasis carbohydrate vaccine. J. Org. Chem. 66, 4233–4243 (2001).
Hewitt, M. C. & Seeberger, P. H. Automated solid-phase synthesis of a branched Leishmania cap tetrasaccharide. Org. Lett. 3, 3699–3702 (2001).
Kannagi, R. et al. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J. Biol. Chem. 258, 8934–8942 (1983).
Danishefsky, S. J. & Allen, J. R. From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew. Chem. Int. Ed. Engl. 39, 836–863 (2000).
Keding, S. J. & Danishefsky, S. J. in Carbohydrate-Based Drug Discovery Vol. 1 (ed. Wong, C.-H.) 381–406 (Wiley-VCH, Weinheim, 2003).
Menard, S., Tagliabue, E., Canevari, S., Fossati, G. & Colnaghi, M. I. Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res. 43, 1295–1300 (1983).
Livingston, P. O. Augmenting the immunogenicity of carbohydrate tumor antigens. Cancer Biol. 6, 357–366 (1995).
Zhang, S. et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer 3, 42–49 (1997).
Kitov, P. I. & Bundle, D. R. Thermodynamic models of the multivalency effect. Carbohydrate-Based Drug Discovery 2, 541–574 (2003).
Karlsson, K. A. Bacterium-host protein carbohydrate interactions and pathogenicity. Biochem. Soc. Trans. 27, 471–474 (1999).
Karlsson, K. A. Pathogen-host protein–carbohydrate interactions as the basis of important infections. Adv. Exp. Med. Biol. 491, 431–443 (2001).
Mammen, M., Choi, S. -K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. Engl. 37, 2745–2794 (1998).
Disney, M. D., Zheng, J., Swager, T. M. & Seeberger, P. H. Detection of bacteria with carbohydrate-functionalized fluorescent polymer. J. Am. Chem. Soc. 126, 13343–13346 (2004).
Disney, M. D. & Seeberger, P. H. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem. Biol. 11, 1701–1707 (2004).
Garegg, P. J. Thioglycosides as glycosyl donors in oligosaccharide synthesis. Adv. Carbohydr. Chem. Biochem. 52, 179–205 (1997).
Kahne, D., Walker, S., Cheng, Y. & van Engen, D. Glycosylation of unreactive substrates. J. Am. Chem. Soc. 111, 6881–6882 (1989).
Koenigs, W. & Knorr, E. Derivatives of grape sugar and galactose. Chem. Ber. 34, 957–981 (1901).
Mukaiyama, T., Murai, Y. & Shoda, S. An efficient method for glucosylation of hydroxy compounds using glucopyranosyl fluoride. Chem. Lett. 431–432 (1981).
Kondo, H. et al. Glycosyl phosphites as glycosylation reagents: scope and mechanism. J. Org. Chem. 59, 864–877 (1994).
Plante, O. J., Palmacci, E. R., Andrade, R. B. & Seeberger, P. H. Oligosaccharide synthesis with glycosyl phosphate and dithiophosphate triesters as glycosylating agents. J. Am. Chem. Soc. 123, 9545–9554 (2001).
Fraser-Reid, B., Konradsson, P., Mootoo, D. R. & Udodong, U. Direct elaboration of pent-4-enyl glycosides into disaccharides. J. Chem. Soc. Chem. Commun. 823–825 (1988).
Seeberger, P. H., Bilodeau, M. T. & Danishefsky, S. Synthesis of biologically important oligosaccharides and other glycoconjugates by the glycal assembly method. J. Aldrichimica Acta 30, 75–92 (1997).
de Paz, J. -L. et al. The activation of fibroblast growth factors by heparin: synthesis, structure, and biological activity of heparin-like oligosaccharides. ChemBioChem 2, 673–685 (2001).
Prabhu, A., Venot, A. & Boons, G. -J. New set of orthogonal protecting groups for the modular synthesis of heparan sulfate fragments. Org. Lett. 5, 4975–4978 (2003).
Orgueira, H. A. et al. Modular synthesis of heparin oligosaccharides. Chem. Eur. J. 9, 140–169 (2003). A highly flexible modular approach for the construction of heparin-like structures is presented.
Shukla, D. et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99, 13–22 (1999).
Acknowledgements
D.B.W. thanks the Alexander von Humboldt Foundation (AvH) for a Feodor Lynen Research Fellowship and the Deutsche Forschungsgemeinschaft (DFG) for a Emmy Noether Fellowship. P.H.S. thanks the Swiss Federal Institute of Technology (ETH) and the Swiss National Science Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.H.S. holds stock in Ancora Pharmaceuticals, a company that utilizes automated oligosaccharide synthesis in drug discovery.
Related links
Glossary
- GENOMICS AND PROTEOMICS
-
All aspects dealing with the biology of nucleic acids and proteins. Analogous, for all aspects dealing with glycobiology the term 'glycomics' was introduced.
- POLYMERASE CHAIN REACTION (PCR)
-
The purpose of this enzymatic reaction is to make a huge number of copies of a gene. This is necessary to have enough starting template for sequencing.
- PROTECTING GROUP
-
A chemical moiety that is introduced to prevent a reaction at a certain position. Protecting groups can be permanent or temporary. The former remain during the complete synthesis in the molecule, whereas the latter protect the functional group only during some steps. Protecting groups should also be removable in an easy fashion.
- ONE-POT METHOD
-
Sequence of reactions carried out in the same reaction vessel without purifying intermediate compounds.
- SOLID-PHASE CHEMISTRY
-
In solid-phase synthesis, the compounds being synthesized are attached (usually by a linker moiety) to an insoluble polymeric material (usually beads or resin), allowing the reaction products to be readily separated (for example, by filtration or centrifugation) from excess reagent, soluble reaction by-products and solvents.
- ANTIGEN
-
Antigens are macromolecules that elicit an immune response in the body. Antigens can be proteins, conjugates of lipids with proteins (lipoproteins) or polysaccharides (glycolipids).
- SELECTINS
-
A family of carbohydrate-binding proteins (lectins) expressed on activated platelets (P-selectin) and endothelial cells (P- and E-selectin).
- EPITOPE
-
A part of an antigen to which an antibody binds. Also called an antigenic determinant.
- MICROARRAY
-
A chip, prepared by attachment of biopolymers to a surface in a spatially discrete pattern. Microarrays have enabled a low-cost and high-throughput methodology to screen interactions of molecules.
- α-GLUCOSIDASE
-
Carbohydrate-splitting enzymes that catalyse the hydrolysis of α-glucosidic linkages in oligo- and polysaccharides.
- KEYHOLE LIMPET HAEMOCYANIN
-
A high-molecular-mass polymeric glycoprotein derived from the haemolymph of the giant sea mollusk Megathura crenulata and frequently used as a highly immunogenic carrier for carbohydrate antigens.
Rights and permissions
About this article
Cite this article
Seeberger, P., Werz, D. Automated synthesis of oligosaccharides as a basis for drug discovery. Nat Rev Drug Discov 4, 751–763 (2005). https://doi.org/10.1038/nrd1823
Issue Date:
DOI: https://doi.org/10.1038/nrd1823
This article is cited by
-
Automated synthesis of prexasertib and derivatives enabled by continuous-flow solid-phase synthesis
Nature Chemistry (2021)
-
Synthesis of glycoconjugates utilizing the regioselectivity of a lytic polysaccharide monooxygenase
Scientific Reports (2020)
-
Towards the generalized iterative synthesis of small molecules
Nature Reviews Chemistry (2018)
-
New agents in HSC mobilization
International Journal of Hematology (2017)
-
Synthesis of Glycosylated Chrysin Derivatives Via Ester Linkers
Chemistry of Natural Compounds (2016)