Key Points
-
Despite progress in antiplatelet therapy, a large amount of unmet clinical need continues to exist. According to current estimates, >60 million people in the United States alone have one or more forms of cardiovascular disease and a high proportion of these individuals are at an increased risk of arterial thrombosis.
-
Antithrombotics are the most rapidly growing sector of the cardiovascular market, with sales around US $9.1 billion in 2001 that are forecast to grow to US $22 billion by the year 2007. One of the major factors underpinning this growth is the rapid increase in sales of the antiplatelet drug clopidogrel, which are increasing by an average of 65% per year at present.
-
Although aspirin reduces acute vascular events in approximately 25% of patients with vascular disease, the residual risk remains high, justifying the need to develop more effective antithrombotic agents or use combination antiplatelet therapies.
-
An important lesson that has emerged from numerous antithrombotic trials is that increased antithrombotic potency per se does not necessarily guarantee enhanced clinical benefit and that, in general, potent antithrombotic approaches must be reserved for high-risk patient populations.
-
Bleeding represents the most important factor influencing long-term compliance and overall clinical benefit of antithrombotic therapy. The key concern regarding all existing antithrombotic drugs relates to their therapeutic window. Ideally, antithrombotic approaches should target one or more processes that are crucial for pathological thrombosis, but which are less important for haemostasis. Despite intensive effort, major progress in this area has been limited.
-
Novel antithrombotic approaches are likely to evolve from an improved understanding of the mechanisms regulating thrombus formation. In this context, there have been considerable recent advances, largely gained from the development of novel experimental techniques enabling real-time analysis of arterial thrombus formation in vivo.
-
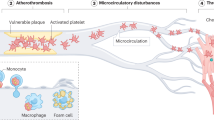
The factors contributing to an exaggerated platelet response at sites of atherosclerotic plaque rupture are multifactorial: the net result of changes in platelet reactivity; plaque thrombogenicity; rheological disturbances and the breakdown of the normal control mechanisms dampening platelet activation.
-
Future advances in antiplatelet therapy are likely to derive from improvements in the understanding of the molecular events regulating thrombogenesis. In this context, the determination of factors specifically promoting an exaggerated platelet response at sites of atherosclerotic plaque rupture could provide a rational basis for the design and development of novel antiplatelet agents with improved efficacy/side-effect profiles.
Abstract
The central importance of platelets in the development of arterial thrombosis and cardiovascular disease is well established. No other single cell type is responsible for as much morbidity and mortality as the platelet and, as a consequence, it represents a major target for therapeutic intervention. The growing awareness of the importance of platelets is reflected in the increasing number of patients receiving antiplatelet therapy, a trend that is likely to continue in the future. There are, however, significant drawbacks with existing therapies, including issues related to limited efficacy and safety. The discovery of a 'magic bullet' that selectively targets pathological thrombus formation without undermining haemostasis remains elusive, although recent progress in unravelling the molecular events regulating thrombosis has provided promising new avenues to solve this long-standing problem.
Similar content being viewed by others
Main
Excessive accumulation of platelets at sites of atherosclerotic plaque rupture is a crucial pathogenic event that is responsible for the development of the acute coronary syndromes, stroke and the ischaemic complications of peripheral vascular disease1,2. Progress in the understanding of the central importance of platelets in cardiovascular disease, combined with the development of new classes of antiplatelet agents, has dramatically altered the landscape in terms of the clinical management of vascular diseases. Until recently, aspirin was the only antiplatelet agent in widespread clinical use for the prevention and treatment of cardiovascular disease. Although it continues to remain the gold-standard antiplatelet therapy, ADP-receptor antagonists and phosphodiesterase (PDE) inhibitors are increasingly recognized as important therapeutic options in high-risk patient populations3. The recent emergence of antagonists of the glycoprotein (GP) GPIIb–IIIa (also known as integrin αIIbβ3) has heralded a new era in antiplatelet therapy and provided clinical confirmation of the importance of platelets in the acute and chronic complications of various cardiovascular diseases. The enthusiasm with which the GPIIb–IIIa antagonists have been embraced by the medical, pharmaceutical and academic communities is indicative of their market potential, and of the clinical desire for more effective antithrombotic approaches. Despite the progress that has been made in the development of antiplatelet therapies, there is still a large unmet clinical need for new treatments. On the basis of current estimates from the American Heart Association, more than 60 million people in the United States alone have one or more forms of cardiovascular disease, and a high proportion of these individuals are at increased risk of arterial thrombosis. There is, however, some cause for optimism, particularly in light of the rapidly evolving understanding of the molecular events governing haemostasis and thrombosis, and the plethora of potential new therapeutic targets that are emerging.
An excellent review on the current status of antiplatelet therapy has recently been published in this journal3, and several extensive reviews on aspirin4,5, thienopyridine antagonists3,6,7,8,9,10, PDE11,12,13 and GPIIb–IIIa antagonists3,14,15,16 have also been published. In the current review, we will discuss the clinical requirement for more effective antithrombotic therapies, and highlight limitations of existing therapeutic strategies. We will also provide an update on recent advances in the understanding of the role of platelets in thrombosis and the molecular mechanisms involved in thrombus formation. Finally, we will discuss important differences between physiological haemostatic plug formation and pathological thrombosis, highlighting potential future approaches that might reduce pathological thrombus formation while minimizing the impact on haemostasis.
Growing clinical need for antithrombotic therapy
A recent meta-analysis of more than 287 antithrombotic clinical trials has demonstrated the benefit of antiplatelet therapy in a diverse range of cardiovascular diseases (Table 1)17. These include patients presenting acutely with myocardial infarction, ischaemic stroke or unstable angina, and also patients with a past history of previous myocardial infarction or cerebrovascular ischaemic events. Antiplatelet therapy has also proven beneficial for primary prevention in patients with peripheral arterial disease and atrial fibrillation, and reduces serious vascular events in other high-risk individuals, including diabetics, patients with end-stage renal disease requiring fistula or shunt placement, and individuals with symptomatic or asymptomatic carotid stenosis17.
The increasing use of antithrombotic therapy is reflected by recent trends in global sales of these products. Antithrombotics are the most rapidly growing sector of the cardiovascular market, and sales of around US $9.1 billion in 2001 are forecast to grow to US $22 billion by 2007 (Ref. 18). One of the principal factors underpinning this growth is the rapid increase in sales of the antiplatelet drug clopidogrel, which are presently increasing by an average of 65% per year (Fig. 1). This dramatic growth reflects the expansion of the clinical indications for which the drug is approved and a favourable safety profile relative to other antiplatelet agents, including ticlopidine.
Data taken from Ref. 18. PDE, phosphodiesterase.
Several factors are likely to result in further expansion of the antithrombotic market, including the ageing population with its attendant increase in vascular pathology; the global increase in the incidence of cardiovascular disease due to alterations in diet and lifestyle; the increased recognition by clinicians of the importance of antiplatelet therapy in the prevention and treatment of cardiovascular diseases; the growing trend of combining antiplatelet therapies in patients at high risk of arterial thrombosis; and the increasing number of patients requiring secondary thromboprophylaxis as a result of improvements in survival from acute myocardial infarction.
Efficacy versus safety
The two most widely used oral antiplatelet drugs, aspirin and clopidogrel, are generally well tolerated and produce significant clinical benefit in a broad range of patient populations. The major limitation of these drugs is their relatively weak antithrombotic effect. For example, extensive clinical studies have demonstrated that despite reducing the incidence of acute myocardial infarction and stroke by 34% and 25%, respectively, aspirin only prevents vascular death in ∼15% of patients17. Clopidogrel is only slightly better, producing a 9% risk reduction in serious vascular events relative to aspirin17,19. It should be noted that in experimental models, clopidogrel has considerably more potent antithrombotic activity than aspirin when administered at high concentrations. However, in most clinical studies, clopidogrel has been used at concentrations that inhibit ADP-induced platelet aggregation by ∼40–50%, as higher levels of receptor blockade lead to a marked increase in BLEEDING TIME20.
The limited efficacy of aspirin and ADP-receptor antagonists reflects the existence of alternative pathways for platelet activation (Figs 2 and 3). Platelets are exposed to a diverse range of activating stimuli at sites of vascular injury, including adhesive proteins (for example, fibrillar collagens, von Willebrand factor (vWf) and fibronectin) and soluble agonists (for example, thrombin, ADP, thromboxane A2 (TXA2) and serotonin) that induce platelet activation through well-defined receptor-coupled signalling pathways21 (Fig. 3). Ultimately, these pathways converge on the activation of GPIIb–IIIa, the major platelet adhesion receptor, which has a pivotal role in promoting platelet aggregation and thrombus growth22,23,24. This major platelet receptor represents 'the final common pathway' for platelet aggregation, regardless of the primary thrombogenic stimulus, thereby providing the rationale for the development of GPIIb–IIIa inhibitors. In extensive clinical studies, GPIIb–IIIa inhibitors have proven to be a powerful class of antiplatelet agents and provide significant clinical benefit in patients undergoing coronary interventions25. However, similarly to the thienopyridines (ticlopidine and clopidogrel), the high level of receptor blockade required to achieve a potent antithrombotic effect significantly undermines haemostasis, resulting in an increased risk of bleeding26.
The principal factor regulating the adhesiveness of platelets is the activation state of GPIIb–IIIa. The affinity status of this receptor is strictly regulated by a balance of activating (+ve; ADP, thrombin, TXA2) and inhibitory signals (-ve; prostacyclin, NO). A number of these regulatory pathways have been successfully targeted therapeutically, leading to the development of a diverse range of antithrombotic approaches. These include various surface-receptor antagonists (ADP P2Y12 receptor: ticlopidine and clopidogrel; GPIIb–IIIa: abciximab, tirofiban and eptifibatide), inhibitors of platelet signalling enzymes (COX: aspirin; cAMP PDE: cilostazol; cGMP PDE: dipyridamole), receptor agonists (prostacyclin: iloprost) and soluble agonist inhibitors (thrombin: heparins, direct thrombin inhibitors or vitamin K antagonists). *Note: the antithrombotic effects of thrombin antagonists are primarily due to the inhibition of fibrin generation, although they can also indirectly dampen platelet activation. Also, it should be noted that although dipyridamole is primarily a cGMP PDE inhibitor, it also indirectly affects cAMP levels and inhibits cellular uptake and metabolism of adenosine, leading to further inhibition of platelet function. The efficacy of individual drug therapies (see Box 1) is influenced by a number of factors, including the clinical indication for antithrombotic therapy, patient responsiveness to the therapeutic agent as well as drug pharmacokinetics and pharmacodynamics. For example, although aspirin and ADP-receptor antagonists inhibit two important pathways for platelet activation, other alternative pathways — that is, downstream of thrombin receptors — can bypass the effects of these drugs and induce GPIIb–IIIa activation and thrombosis. Direct GPIIb–IIIa antagonists overcome this limitation, producing potent antithrombotic effects. AA, arachidonic acid; COX, cyclooxygenase; NO, nitric oxide; PDE, phosphodiesterase; TXA2, thromboxane A2.
Schematic representation of the major adhesion and agonist receptors on the surface of platelets. On the basis of mouse gene-knockout studies and platelets from patients with various haemostatic disorders, the specific contribution of many of these receptors to haemostasis and thrombosis has been well defined. GPIb/V/IX: binds vWf expressed on the subendothelium and on the surface of platelets. This receptor also potentiates platelet activation in response to low-dose thrombin, and is important in mediating platelet adhesion and aggregation under high-shear conditions. GPIIb–IIIa: fundamental for a range of platelet responses, including platelet aggregation, spreading and clot retraction. Binds multiple ligands, including fibrinogen, vWf, polymerized fibrin, fibronectin, vitronectin and soluble CD40 ligands. GPIa–IIa and GPVI: the two major receptors supporting platelet adhesion on collagen. The GPVI receptor is non-covalently associated with the ITAM-bearing receptor, FcR γ-chain. Analysis of mouse platelets lacking GPVI or FcR γ-chain have demonstrated an important role for this receptor complex in supporting platelet adhesion and activation on fibrillar collagens. ADP receptors: two metabotropic receptors for ADP have been identified — P2Y1 and P2Y12. Each ADP receptor is linked to one or more different G-protein(s). Thrombin receptors: thrombin uses three agonist receptors on the surface of platelets. Two of these are the classical protease-activated receptors (PAR1 and PAR4), which are linked to Gi,q,12/13 and Gq,12/13, respectively. The other thrombin receptor is GPIbα. TXA2 receptors: two TXA2 receptors have been identified, TP-α and TP-β, each of which is linked to Gq and G12/13. All of these receptors activate specific signalling cascades that are ultimately linked to the mobilization of calcium from intracellular stores. All soluble agonist receptors utilize G-protein-coupled receptors that induce activation of one or more PLC-β isoforms. Adhesion receptors typically utilize non-receptor tyrosine kinases that regulate cytosolic calcium flux through regulation of PLC-γ isoforms. For clarity, the details of specific signalling events linked to individual receptors have been omitted. Targeting signalling processes linked to the regulation of GPIIb–IIIa have the potential to produce a significant number of novel antithrombotic approaches. GP, glycoprotein; ITAM, immunoreceptor tyrosine-based activating motif; PLC, phospholipase C; TXA2, thromboxane A2; TP, thromboxane/prostanoid receptors; vWf, von Willebrand factor.
The issue of improving efficacy at the expense of safety is a significant problem that plagues all forms of presently available antithrombotic therapy. In the majority of patients with cardiovascular disease, combination antithrombotic therapy is not appropriate for chronic use. In particular, the combined use of antiplatelet and anticoagulant agents has been considered taboo by many clinical centres and reserved only for patients at very high risk of arterial thrombosis. This contrasts with other forms of cardiovascular disease, such as hypertension and cardiac failure, in which multi-component therapy is common, because of the availability of drugs that act in a synergistic manner and which possess a wide THERAPEUTIC WINDOW.
Benefit/risk of combined antithrombotic therapy
Although aspirin reduces acute vascular events in approximately 25% of patients with vascular disease, the residual risk remains high, which justifies the need to develop more effective antithrombotic agents or combination antiplatelet therapies. The success of short-term combination antiplatelet therapies for patients undergoing percutaneous coronary intervention has fuelled interest in the longer-term use of combined approaches. The recent CURE study (Clopidogrel in Unstable Angina to Prevent Recurrent Events)27 showed a significant advantage of combining aspirin with clopidogrel versus aspirin alone (20% relative risk reduction in cardiovascular death, nonfatal myocardial infarction and stroke), in patients with unstable angina or suspected myocardial infarction. As with most combination therapies, this benefit was offset by a corresponding increase in major bleeding (3.7% incidence in the clopidogrel and aspirin group, versus 2.7% in the aspirin-alone group; relative increase 38%). When the antithrombotic benefit of combined therapy is offset by major bleeding events, the overall clinical benefit could be as low as 8%. Whether this combined approach will provide long-term clinical benefit in other cardiovascular patient groups remains to be established, although several major Phase III clinical trials are presently evaluating this approach3.
An important lesson that has emerged from numerous antithrombotic trials is that increased antithrombotic potency per se does not necessarily guarantee enhanced clinical benefit and, in general, potent antithrombotic approaches must be reserved for high-risk patient populations. This has been most clearly demonstrated in clinical trials of the oral GPIIb–IIIa inhibitors, chronic administration of which, particularly at high concentrations, produces an excess of bleeding events (up to 70% incidence of minor bleeding and 12% major bleeds)28. Lower levels of platelet inhibition produce a twofold increase in bleeding risk, although, paradoxically, induce a 35% relative increase in fatal ischaemic events29,30,31. Similarly, several studies of combination antiplatelet and anticoagulant therapies have failed to demonstrate significant clinical benefit, due in part to an increased risk of major bleeds32,33,34. Bleeding represents the most important factor influencing long-term compliance and the overall clinical benefit of antithrombotic therapy. The key concern regarding all existing antithrombotic drugs relates to their therapeutic window. Ideally, antithrombotic approaches should target one or more processes that are crucial for pathological thrombosis, but which are less important for haemostasis. Despite intensive effort, major progress in this area has been limited.
New insights into regulation of thrombus growth
Novel antithrombotic approaches are likely to evolve from an improved understanding of the mechanisms regulating thrombus formation. In this context, there have been considerable recent advances, largely derived from the development of novel experimental techniques that enable the real-time analysis of arterial thrombus formation in vivo35,36,37,38,39,40. In particular, recent developments in intravital imaging techniques have provided unprecedented insights into the complexity and dynamics of thrombus formation. The application of this technology to mouse models in which specific disruptions of one or more haemostatic components have been engineered has redefined our understanding of many basic aspects of the thrombotic process (Tables 2 and 3)41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98.
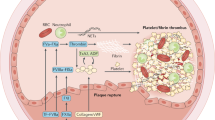
Conceptual advances in the understanding of platelet thrombus growth. The composition of a thrombus is crucially dependent on the RHEOLOGICAL ENVIRONMENT, with arterial thrombi typically being platelet-rich, whereas venous thrombi are platelet-poor and primarily consist of polymerized fibrin and red blood cells. The central role of platelets in arterial thrombus formation is a reflection of the unique ability of these cells to form stable adhesion contacts with the damaged vessel wall under conditions of rapid blood flow. This capacity is largely attributable to the interaction between vWf and its platelet receptor, the GPIb/V/IX complex. The vWf–GPIb interaction has unique biomechanical properties that enable the initiation of platelet adhesive interactions over a remarkably broad range of haemodynamic conditions99. In fact, it is the only known receptor–ligand interaction that can mediate platelet interactions throughout all regions of the vasculature, which explains its unique role in haemostasis and thrombosis. Qualitative or quantitative abnormalities of either vWf or the GPIb/V/IX complex are associated with severe bleeding disorders47,58,59, whereas dysregulated enhancement of this interaction can lead to diffuse microvascular thrombosis, resulting in life-threatening diseases such as THROMBOTIC THROMBOCYTOPAENIC PURPURA (TTP)100.
Platelet adhesion to the vessel wall (primary adhesion) and subsequent platelet aggregation (platelet cohesion) have traditionally been viewed as separate events, involving distinct adhesive ligand–receptor pairs101. The initial adhesion to the damaged arterial vessel wall involves a diverse array of adhesive ligands (for example, vWf, collagen, fibronectin, vitronectin and laminin) and receptors (for example, GPIb, GPVI, and integrins α2β1 (also known as GPIa–IIa), αIIbβ3 (also known as GPIIb–IIIa), α5β1, α6β1 and possibly αvβ3). By contrast, subsequent platelet aggregation has traditionally been thought to involve a single adhesive interaction between soluble fibrinogen and GPIIb–IIIa (Fig. 4). However, the conceptual understanding of platelet aggregation has changed dramatically during the past few years. Platelet aggregation is now considered a complex, dynamic process involving the cooperative adhesive function of multiple adhesive ligands, including vWf, fibrinogen and possibly fibronectin and soluble CD40 ligand, as well as two adhesive receptors, GPIb and GPIIb–IIIa36,38,39,40,102,103,104,105,106,107,108,109,110,111,112,113,114 (Fig. 4). The specific contribution of individual adhesive events to the aggregation process is crucially dependent on the rheological conditions, with the contribution of the vWf–GPIb interaction becoming progressively more important with increasing shear stress (see below). In fact, under high-shear conditions, such as those typically found in small arterioles or stenosed arteries, thrombus growth is primarily mediated by vWf, with fibrinogen playing a secondary role in stabilizing formed aggregates39,113,114.
a | Platelet aggregation has traditionally been viewed as mediated by a single adhesive interaction between GPIIb–IIIa and fibrinogen (suspension). In this model, activation signals emanating downstream of soluble agonist receptors induce a conformational change in the extracellular domain of GPIIb–IIIa, enabling it to engage fluid-phase fibrinogen. The dimeric fibrinogen molecule crosslinks two GPIIb–IIIa receptors on adjacent platelets, thereby mediating platelet aggregation. b | Analysis of thrombus formation in vivo has demonstrated that platelet aggregation is a dynamic process potentially involving multiple ligands and receptors (reactive substrate). Under high-shear conditions, platelet aggregation is reliant on an initial tethering step (tethering), which is dependent on GPIb on the surface of flowing platelets engaging surface-expressed vWf on immobilized platelets. This adhesive interaction also supports platelet rolling (translocation) on the thrombus surface. Subsequent firm adhesion (adhesion and spreading/stable aggregation) is mediated by GPIIb–IIIa engagement of vWf and/or fibrinogen. Recent evidence has indicated that two other potential GPIIb–IIIa ligands, fibronectin and soluble CD40 ligand (not shown), might also participate in arterial thrombogenesis. GP, glycoprotein; GPCR, G-protein-coupled receptor; vWf, von Willebrand factor.
Importance of fibrillar collagens in promoting thrombus growth. Considerable progress has been made during the past five years in unravelling the key molecular events regulating platelet adhesion and thrombus growth on collagen115. These insights are potentially important, as fibrillar collagens type I and III are among the most potent activators of platelets, and their high local concentration in advanced atherosclerotic lesions contributes to the heightened thrombogenic potential of these plaques116. A considerable number of putative collagen receptors have been described on the surface of platelets, although recent evidence indicates that the GPVI receptor is the dominant receptor supporting platelet adhesion and thrombus growth55. GPVI, a member of the immunoglobulin superfamily, is non-covalently associated with the immunoreceptor tyrosine-based activating motif (ITAM)-bearing receptor, the FcR γ-chain117,118. This latter receptor plays a fundamental role in transducing signals downstream of GPVI via signalling pathways similar to those used by other immunoreceptors117,119,120. A deficiency of GPVI leads to a mild bleeding DIATHESIS in humans121, a finding that has been reproduced in experimental mouse models51,52. Immunodepletion of GPVI from the surface of mouse platelets has recently been reported to lead to a profound defect in occlusive thrombus formation in vivo122, although other studies on GPVI123 and FcR γ-chain-deficient mice124 have not demonstrated such a crucial role for this receptor in promoting thrombus formation.
A second well-defined collagen receptor is GPIa–IIa (also known as integrin α2β1). A deficiency of this receptor has been reported to cause a mild bleeding diathesis in humans56,57 and analysis of platelets derived from these individuals in ex vivo perfusion chambers have demonstrated a defect in adhesion and spreading on the subendothelium21,125. Strikingly, in genetically engineered β1-deficient mice lacking α2β1, α5β1 and α6β1, bleeding time is unaffected, and adhesion of these platelets to collagen is normal in extent, albeit delayed relative to normal platelets55. Although the significance of these findings for humans remains to be established, they challenge the long-held belief that GPIa–IIa is the primary collagen receptor mediating platelet adhesion.
Importance of soluble platelet agonists and their receptors in promoting thrombus growth. Efficient platelet activation requires the synergistic contribution of multiple input signals, involving adhesive and soluble stimuli (Fig. 3). The importance of soluble platelet agonists, such as thrombin, ADP and TXA2, in promoting thrombus formation has been clearly demonstrated8,68,69,126,127,128,129,130,131,132,133. These agonists are generated locally at the site of vascular injury and accelerate platelet activation through well-defined receptor-coupled signalling mechanisms8,126,134. However, the contribution of individual agonist receptors to thrombus growth has not been so clearly defined. This is particularly relevant to thrombin and ADP, as each of these agonists can potentially induce platelet activation through multiple surface receptors (ADP through the P2Y1 and P2Y12 receptors; thrombin through protease-activated receptor 1 (PAR1), PAR3 and GPIb)134,135,136. The role of thrombin in arterial thrombosis is further complicated by the myriad effects of this protease on coagulation proteins, fibrinolysis, inflammation and endothelial cells137,138.
Recent insight into the role of ADP and thrombin receptors in arterial thrombosis has been gained from the study of transgenic mouse models68,69,70,71,72,73,74,139,140. These studies have indicated that the P2Y1 and P2Y12 receptors might have distinct but complementary roles in thrombus development136: the P2Y1 receptor promotes initial thrombus growth70,71,72, whereas the P2Y12 receptor primarily serves to sustain the stability of formed thrombi73. Recent studies have also provided evidence for the importance of platelet thrombin receptors in arterial thrombosis. For example, studies of mice lacking either of the major thrombin receptors PAR3 (Ref. 68) or PAR4 (Ref. 69) have demonstrated that both receptors contribute to thrombus formation under high-shear conditions in vivo68,69. Although the protease-activated receptors on the surface of human platelets are distinct from those on mice (PAR1/PAR4 versus PAR3/PAR4), these findings nonetheless provide proof-of-concept validation that thrombin receptor function, distinct from other pro-thrombotic activities of thrombin, is important for arterial thrombosis.
Factors promoting occlusive thrombus formation
The factors contributing to an exaggerated platelet response at sites of atherosclerotic plaque rupture are multifactorial: they are the net result of changes in platelet reactivity, plaque thrombogenicity, rheological disturbances and the breakdown of the normal control mechanisms dampening platelet activation.
Plaque thrombogenicity. Several factors contribute to the heightened thrombogenic potential of atherosclerotic lesions, including high concentrations of fibrillar collagens in the lesion141, tissue factor142,143, as well as the presence of potent platelet-activating lipids, such as lysophosphatidic acid144,145. The presence of collagen in plaques represents a potential double-edged sword. On the one hand, an elevated content of collagen is advantageous as it maintains the strength of the atherosclerotic fibrous cap, thereby preventing plaque rupture146. On the other hand, its potent platelet-activating properties increase the likelihood of forming occlusive thrombi. The importance of tissue factor in promoting plaque thrombogenicity is well established. In advanced lesions, tissue factor is primarily derived from tissue macrophages and T lymphocytes as a consequence of the shedding of apoptotic pro-coagulant microparticles147. Studies demonstrating that direct inhibition of tissue factor markedly reduces thrombus growth on ruptured atherosclerotic plaques143 provide strong experimental support for the rationale of using antithrombin agents in the management of the acute coronary syndromes.
Platelet reactivity. Enhanced platelet reactivity is likely to contribute to occlusive thrombus formation in high-risk patient groups. Patients with hyper-reactive platelets are more likely to have acute coronary events148,149, and diabetics in particular (who have more reactive platelets) benefit from more intensive antiplatelet therapy than do other patient groups150,151. The mechanisms responsible for platelet hyper-reactivity are incompletely understood, but probably reflect the contribution of various congenital and acquired influences. Increased platelet reactivity has been reported in individuals with hypertension152,153, diabetes153 and hypercholesterolaemia154, and in cigarette smokers155 and older age groups156. Dietary factors, such as alcohol157, dietary fats, fish and fish oils158, have also been reported to regulate platelet responsiveness. There is growing interest in the possible involvement of glycoprotein polymorphisms in regulating the reactivity of platelets159 and, although controversial, there is evidence that polymorphisms in GPIb160,161,162, GPIIb–IIIa163 and GPIa–IIa164 increase the risk of arterial thrombosis.
Disturbed rheology. Rheological disturbances at sites of arterial stenosis are important in promoting occlusive thrombus formation21. Platelets are exposed to high-shear forces and a range of abnormal flow patterns during the atherothrombotic process, including flow separation, eddy formation, flow reversal and turbulence, which produce complex, rapid changes in the shear environment165,166,167. Despite their importance for thrombosis, the mechanism(s) by which shear forces induce platelet activation remains incompletely understood. On the basis of available experimental evidence, shear-induced platelet activation is crucially dependent on the adhesive interaction between vWf, GPIb and GPIIb–IIIa, with an important role for released ADP in potentiating platelet activation112,168,169,170,171. It is noteworthy that aspirin is unable to inhibit the effects of shear on platelet activation, a finding that might partially explain its limited clinical efficacy169,172,173.
New approaches to antiplatelet therapy
Future advances in antiplatelet therapy are likely to derive from improvements in the understanding of the molecular events regulating thrombogenesis. In this context, the elucidation of factors specifically promoting an exaggerated platelet response at sites of atherosclerotic plaque rupture could provide a rational basis for the design and development of novel antiplatelet agents with improved efficacy/side-effect profiles. In addition, a considerable number of potentially new antithrombotic approaches are likely to emerge as a direct result of knockout mouse studies (Tables 2 and 3). The usefulness of the latter approach for proof-of-concept validation of pharmaceutical products is well established174. In the remainder of this review, we will discuss potential novel approaches for antiplatelet therapy, focusing on targets for which there is experimental evidence that their inhibition produces an antithrombotic effect in vivo. On the basis of current knowledge, three distinct groups of antiplatelet drugs are likely to emerge in the future: anti-adhesive drugs; drugs that can directly inhibit agonist receptors; and drugs that target one or more intracellular signalling pathways linked to platelet activation.
Anti-adhesive drugs. There are potentially a large number of adhesive proteins and receptors contributing to platelet thrombus growth; however, in only a few cases — specifically, fibrinogen, vWf and collagen — is there strong evidence for their involvement in occlusive thrombus formation. The importance of blocking fibrinogen binding to GPIIb–IIIa is well established and will not be discussed further here.
Inhibiting the vWf–GPIb interaction. The growing recognition of the importance of the vWf–GPIb interaction for both primary platelet adhesion and aggregation, particularly under high-shear conditions, has focused considerable attention on this receptor as a potential antithrombotic target. Deficiency of GPIb in humans and mice leads to a severe bleeding diathesis46,47, underscoring the importance of this receptor for normal haemostasis; however, studies in mouse models indicate that the haemostatic defect is not as severe as deficiency of GPIIb–IIIa41,42,43,46, raising the possibility that inhibitors of GPIb might have better long-term tolerance than GPIIb–IIIa inhibitors. It is worth noting that platelets lacking the GPIb complex have other abnormalities beyond defective vWf binding, including THROMBOCYTOPAENIA, an abnormal morphology, decreased responsiveness to thrombin and a reduced pro-coagulant function46,47, all of which could contribute to the increased bleeding risk. Antibodies against GPIb or vWf have potent antithrombotic activity in vivo44,45,175,176,177,178,179, and, in contrast to GPIIb–IIIa inhibitors, can inhibit thrombus growth at concentrations that do not increase bleeding time45,180. Furthermore, given that the GPIb-α subunit is primarily expressed on platelets, such inhibitors are likely to have minimal non-haemostatic side-effects. There is growing evidence for the importance of the GPIb subunit in supporting platelet–leukocyte interactions, owing to its ability to act as a counter-receptor to one or more leukocyte and endothelial cell-adhesion molecules181,182,183. Given the growing awareness of the importance of inflammation in influencing the acute and chronic outcomes of cardiovascular disease184,185, GPIb inhibitors that dampen platelet–leukocyte interactions might have additional therapeutic benefits. Nonetheless, there are significant challenges associated with the development of GPIb inhibitors, not least their probable deleterious effects on haemostasis, problems associated with antibody-induced thrombocytopaenia186,187 and potential pathogenic effects on megakaryocytes188,189. The development of GPIb inhibitors is also hampered by the poor cross-species reactivity of anti-GPIb antibodies and by the lack of well-defined small-molecule PHARMACOPHORES necessary for the generation of orally bioavailable compounds. Moreover, the evaluation of these inhibitors in early-stage proof-of-concept clinical trials will be a challenge, particularly in patients with coronary disease, due to the number of competing antithrombotic agents already available in the clinic.
Inhibiting collagen–platelet interactions. Progress in elucidating the key adhesive receptors mediating platelet–collagen interactions, combined with the perceived importance of these interactions for arterial thrombosis, has focused considerable recent interest on the development of antagonists of collagen receptors. The case for developing such inhibitors is further strengthened by clinical and experimental evidence that a deficiency of either of the two major collagen receptors GPVI and GPIa–IIa produces only a modest defect in haemostasis53,57,125. It should be noted that in addition to supporting platelet adhesion directly, collagen also facilitates platelet–vessel wall interactions indirectly by providing binding sites for vWf. The interaction between collagen and vWf is particularly important under conditions of rapid blood flow, such as those typically found in the arteriolar circulation and at sites of arterial stenosis102. The binding site on vWf for collagen has been localized to the A3 domain of vWf, and specific disruption of this interaction has been shown to lead to reduced thrombus growth in vivo190. Validation of the importance of these individual adhesive interactions for thrombus growth, particularly in diseased atherosclerotic arteries, has been hampered by the lack of suitable experimental models. This is potentially important, given that collagen antagonists would be the first antithrombotic approach that targets primary platelet adhesion, rather than aggregation. So the predictive power of any given preclinical thrombosis model will be greatly influenced by the nature of the thrombogenic surface exposed following vascular injury. A further potential limitation of selective inhibitors of platelet adhesion is that they might afford limited antithrombotic protection in patients with established arterial thrombosis, including patients with unstable angina, myocardial infarction and ischaemic stroke. In this context, it is relevant that a high proportion of patients presenting with an acute myocardial infarction have evidence of sub-acute thrombus formation at the culprit lesion for up to a week before the clinical event191. So the clinical utility of antagonists of collagen receptors might be restricted to specific subsets of patients with vascular disease.
Drugs that can directly inhibit agonist receptors. There has been long-standing interest in attempting to improve on the antithrombotic effect of aspirin. Inhibition of cyclooxygenase-1 prevents the conversion of arachidonic acid (AA) to prostaglandin endoperoxides and thromboxane A2 (TXA2), thereby eliminating an important autofeedback mechanism that serves to amplify platelet activation192,193. The antithrombotic effect of inhibiting TXA2 is partially offset by inhibition of another arachidonic acid metabolite, prostacyclin (PGI2), a powerful endothelial-derived inhibitor of platelets. Strategies to eliminate the platelet-activating properties of TXA2 without affecting PGI2 function have involved developing combined inhibitors of TXA2 synthase and the TXA2 receptor194,195,196,197. Despite showing considerable promise in preclinical studies, these newer approaches have been disappointing in clinical trials, and have not demonstrated a benefit over aspirin.
The success of the ADP-receptor antagonists in improving clinical outcomes has fuelled interest in the possible inhibition of other agonist receptors. Confirmation of the role of the ADP P2Y1 receptor, and the two thrombin receptors PAR3 and PAR4, in promoting thromboembolism has been gained through the study of knockout mouse models. However, in these mice, blunting the platelet responses to ADP and thrombin leads to an increased tail-bleeding time68,69,70,71. The clinical significance of these findings remains uncertain due to the paucity of patients identified with a bleeding tendency linked to deficiency of these receptors. This contrasts with the P2Y12 receptor, a deficiency of which in humans leads to a mild bleeding tendency74. An important outstanding issue with regards to PAR1-, PAR4- and P2Y1-receptor antagonists relates to the potency of their antithrombotic effect relative to P2Y12-receptor antagonism and whether these approaches offer an improved benefit-to-bleeding risk ratio over existing therapies.
Drugs that target one or more signalling pathways linked to platelet activation. The importance of various signalling cascades in regulating platelet function is becoming increasing well understood. Soluble agonists induce platelet activation through distinct G-protein-coupled receptors linked to the regulation of phospholipase C (PLC), phosphatidylinositol 3-kinase (PI3K), adenylyl cyclase and the Rho family of GTPases, whereas platelet adhesion receptors typically initiate platelet activation through one or more non-receptor tyrosine kinases21. There are a large number of knockout mouse models available with specific signalling defects, and detailed analyses of these mice will probably lead to the identification of novel antithrombotic targets. Several signalling enzymes have been identified, such as Gαq and PLC-γ2, which have an essential role in normal platelet function198 and their deletion is associated with a marked bleeding tendency86,93. However, other signalling enzymes, such as the γ-isoform of PI3K, seem to be involved in promoting thrombus formation but are less crucial for normal haemostasis81.
Miscellaneous targets. A number of promising antithrombotic targets that have an incompletely defined role in platelet function have been discovered as a direct result of mouse transgenic technology. In several examples, mice have a defect in thrombus formation with minimal prolongation of tail-bleeding time (Tables 2 and 3). These include growth arrest-specific 6 (Gas6), a previously unrecognized platelet protein that seems to be important in potentiating platelet activation and thrombus growth97; the soluble form of CD40 ligand, a principal mediator of inflammation that seems to be important in regulating thrombus stability through its ability to engage GPIIb–IIIa98,199; and P-selectin, a major platelet α-granule protein that is crucial in promoting platelet–leukocyte interactions and fibrin accumulation63,64.
A universal antithrombotic — myth or reality?
So, what are some of the likely targets for the development of the 'magic bullet'? Before attempting to answer this question, some consideration is required as to the clinical context of antithrombotic therapy. In general, it has proven difficult to develop drugs that are universally effective in all patients with cardiovascular disease, as the specific requirements of antithrombotic drugs are partially dependent on the clinical circumstances of their use. For example, the antithrombotic needs of a patient presenting to hospital with an acute ischaemic stroke are markedly different from those of a patient with stable coronary artery disease requiring long-term antiplatelet therapy. With the former, the antithrombotic approach must have a rapid onset of effect, must have minimal effect on bleeding risk (due to the risk of intracerebral haemorrhage) and must be compatible with thrombolytic agents. Ideally, such an agent would also have anti-inflammatory properties, thereby reducing neuronal damage associated with ischaemia-reperfusion injury. Fulfilling such stringent criteria will be a challenge, particularly for a single therapeutic agent. Similarly, patients undergoing coronary artery bypass surgery or CAROTID ENDARTERECTOMY have a relatively high incidence of thromboembolic events200,201, and the need for an antiplatelet agent that does not increase surgical bleeding remains high. In other acute situations, such as PERCUTANEOUS CORONARY INTERVENTION and unstable angina, bleeding concerns are less crucial (although still important), and combination therapies with potent antithrombotic properties are generally well tolerated and provide significant clinical benefit. In the future, long-term antiplatelet therapies will need to demonstrate improved efficacy, cause fewer bleeding side-effects and, ideally, not increase bleeding risk when combined with other antithrombotic agents.
Which platelet adhesion receptors/ligands, when inhibited, will most likely lead to improvements in antithrombotic therapy? It is our view that GPIb is likely to be the best adhesion-receptor target for the development of novel antithrombotic agents, at least for the management of acute thrombotic events. As with GPIIb/IIIa, GPIb is the only platelet adhesion receptor that has a clear-cut non-redundant role in haemostasis and thrombosis, with a haemostatic defect that is less severe than that associated with deficiency of GPIIb–IIIa. As stated earlier, this defect might be less apparent with selective inhibition of the vWf–GPIb interaction, particularly if thrombin binding and other haemostatic functions of GPIb are preserved. Also, the potential to develop inhibitors that preferentially block platelet adhesion under pathological shear conditions is also an attractive feature of this target. In short, GPIb inhibitors will almost certainly have a wider therapeutic index than GPIIb–IIIa inhibitors, although it is uncertain whether this will be wide enough to truly define these inhibitors as magic bullets. It is less likely that targeting of any other single adhesion receptor will produce as potent an antithrombotic effect as GPIb or GPIIb–IIIa inhibitors, primarily because of the degree of redundancy among receptors involved in primary platelet adhesion. On a more positive note, blocking platelet collagen receptors might cause less severe bleeding complications, thereby improving their overall tolerance and clinical utility. Despite the obvious appeal of targeting collagen–vWf and collagen–platelet adhesive interactions, there remains a considerable amount of work to be done to more clearly establish the therapeutic potential of such approaches.
What agonist receptors are likely to challenge the TXA2 and P2Y12 receptors as preferred targets for novel antithrombotics? This is a difficult question to answer with the current level of experimental and clinical information, although obvious candidates include the P2Y1, PAR1 and PAR4 receptors. On the basis of human and animal studies, blocking the platelet P2Y12 receptor causes a marked defect in haemostasis, underscoring the importance of this receptor for normal platelet function, and, as a consequence, the need to develop drugs with highly predictable pharmacokinetic properties. To date, P2Y1, PAR1 and/or PAR4 antagonists with sufficient specificity and potency for clinical evaluation have yet to emerge; however, on the basis of the findings from knockout mice, it seems that these inhibitors might have less of an impact on haemostasis. It is noteworthy that the platelet inhibitors that presently enjoy the greatest clinical success — aspirin and thienopyridines — primarily inhibit pathways that amplify platelet activation. In this context, the finding that Gas6-deficient platelets are less responsive to stimulation by conventional stimuli and have defective thrombus growth in vivo makes it a target of considerable therapeutic interest.
Unravelling the key signalling mechanisms regulating the adhesive function of GPIIb–IIIa remains a major research objective that could ultimately lead to the development of entirely new classes of antithrombotic agents. In this regard, developments in mouse transgenic technology are likely to feature prominently, and several interesting platelet phenotypes have already begun to emerge as a consequence of defective bi-directional GPIIb–IIIa signalling. A potential benefit of inhibiting signalling targets, particularly those that converge from multiple input signals, is the ability to control overall platelet reactivity, regardless of the nature of the primary thrombogenic stimulus. Furthermore, signalling pathways that are shared by one or more haemopoietic cell types offer the opportunity of developing drugs that have both antiplatelet and anti-inflammatory activity. A novel approach that has yet to be exploited therapeutically is the targeting of shear-sensitive (that is, mechanosensory) signalling pathways in platelets. These pathways are likely to operate, at least in part, downstream of the two major shear-regulated adhesion receptors, GPIb and GPIIb–IIIa. The challenge of such an approach is to identify the crucial signalling elements required for shear activation of platelets and thrombosis, but which are less important for haemostasis. Ideally, these targets would have limited tissue distribution, or alternatively, inhibitors with reduced systemic effects would be employed, that is, aspirin-like drugs (irreversible inhibitors with a short plasma half-life).
Conclusions
The discovery of a 'magic bullet' that selectively targets pathological thrombi has proven elusive, primarily because the molecular events regulating thrombosis are in large part identical to those underlying haemostasis. In fact, it remains to be proven whether there are indeed true 'pathogenic' mechanisms operating during thrombus formation that are not involved in haemostasis. This said, there are clearly important differences between the two processes, and future strategies aimed at exploiting these differences might lead to novel therapies with improved safety and efficacy profiles. It remains uncertain whether it will ever be possible to develop a single drug that will be universally effective at inhibiting pathological thrombi. It is perhaps more probable that future advances will involve the development of combination therapies targeting one or more steps in the thrombotic process. Such multi-component therapy will need to take into consideration the complex interplay between platelets and proteins of the coagulation, fibrinolytic and inflammation systems, although undoubtedly platelet inhibitors will continue to take centre stage in the antithrombotic arena.
References
Fuster, V., Badimon, L., Badimon, J. J. & Chesebro, J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N. Engl. J. Med. 326, 242–250 (1992).
Falk, E., Shah, P. K. & Fuster, V. Coronary plaque disruption. Circulation 92, 657–671 (1995).
Bhatt, D. L. & Topol, E. J. Scientific and therapeutic advances in antiplatelet therapy. Nature Rev. Drug Discov. 2, 15–28 (2003). A comprehensive and up-to-date review on the current status of antiplatelet therapy.
Schror, K. Antiplatelet drugs. A comparative review. Drugs 50, 7–28 (1995).
Awtry, E. H. & Loscalzo, J. in Platelets (ed. Michelson, A. D.) 745–759 (Academic, San Diego, USA, 2002).
Solet, D. J., Zacharski, L. R. & Plehn, J. F. The role of adenosine 5′-diphosphate receptor blockade in patients with cardiovascular disease. Am. J. Med. 111, 45–53 (2001).
Becker, R. C. Platelet surface physiology and its importance in pharmacotherapy design and development: the adenosine diphosphate receptor antagonists. J. Thromb. Thrombolysis. 10, 35–53 (2000).
Gachet, C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 86, 222–232 (2001).
Sharis, P. J., Cannon, C. P. & Loscalzo, J. The antiplatelet effects of ticlopidine and clopidogrel. Ann. Intern. Med. 129, 394–405 (1998).
Curtin, R., Cox, D. & Fitzgerald, D. in Platelets (ed. Michelson, A. D.) 787–797 (Academic, San Diego, USA, 2002).
Movsesian, M. A. Therapeutic potential of cyclic nucleotide phosphodiesterase inhibitors in heart failure. Expert Opin. Investig. Drugs 9, 963–73 (2000).
Ikeda, Y., Sudo, T. & Kimura, Y. in Platelets (ed. Michelson, A. D.) 817–820 (Academic, San Diego, USA, 2002).
Eisert, W. G. in Platelets (ed. Michelson, A. D.) 803–810 (Academic, San Diego, USA, 2002).
Casserly, I. P. & Topol, E. J. Glycoprotein IIb/IIIa antagonists — from bench to practice. Cell. Mol. Life Sci. 59, 478–500 (2002).
Kam, P. C. & Egan, M. K. Platelet glycoprotein IIb/IIIa antagonists: pharmacology and clinical developments. Anesthesiology 96, 1237–1249 (2002).
Agah, A., Plow, E. F. & Topol, E. J. in Platelets (ed. Michelson, A. D.) 769–781 (Academic, San Diego, USA, 2002).
Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002). The largest ever meta-analysis of antiplatelet clinical trials demonstrating the importance of antiplatelet therapy in a wide range of cardiovascular diseases.
Birch, S. The Cardiovascular Outlook to 2007 (Datamonitor, PLC, Reuters Business Insight, London, UK, 2002).
A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348, 1329–1339 (1996). A landmark study demonstrating the efficacy and safety of clopidogrel relative to aspirin in a diverse group of patients with cardiovascular diseases.
Thebault, J. J., Kieffer, G., Lowe, G. D., Nimmo, W. S. & Cariou, R. Repeated-dose pharmacodynamics of clopidogrel in healthy subjects. Semin. Thromb. Hemost. 25 (Suppl. 2), 9–14 (1999).
Ruggeri, Z. M. Platelets in atherothrombosis. Nature Med. 8, 1227–1234 (2002).
Phillips, D. R., Charo, I. F. & Scarborough, R. M. GPIIb-IIIa: the responsive integrin. Cell 65, 359–362 (1991).
Du, X. & Ginsberg, M. H. Integrin αIIbβ3 and platelet function. Thromb. Haemost. 78, 96–100 (1997).
Plow, E. F., D'Souza, S. E. & Ginsberg, M. H. Ligand binding to GPIIb-IIIa: a status report. Semin. Thromb. Hemost. 18, 324–332 (1992).
Casserly, I. P. & Topol, E. J. Glycoprotein IIb/IIIa antagonists- from bench to practice. Cell. Mol. Life Sci. 59, 478–500 (2002).
Scarborough, R. M., Kleiman, N. S. & Phillips, D. R. Platelet glycoprotein IIb/IIIa antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation 100, 437–444 (1999).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Harrington, R. A. et al. Dose-finding, safety, and tolerability study of an oral platelet glycoprotein IIb/IIIa inhibitor, lotrafiban, in patients with coronary or cerebral atherosclerotic disease. Circulation 102, 728–735 (2000).
Chew, D. P., Bhatt, D. L., Sapp, S. & Topol, E. J. Increased mortality with all platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation 103, 201–206 (2001).
Newby, L. K. & McGuire, D. K. Oral platelet glycoprotein IIb/IIIa inhibition. Curr. Cardiol. Rep. 2, 372–377 (2000).
Chew, D. P., Bhatt, D. L., Sapp, S. & Topol, E. J. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of Phase III multicenter randomized trials. Circulation 103, 201–206 (2001).
Huynh, T., Theroux, P., Bogaty, P., Nasmith, J. & Solymoss, S. Aspirin, warfarin, or the combination for secondary prevention of coronary events in patients with acute coronary syndromes and prior coronary artery bypass surgery. Circulation 103, 3069–3074 (2001).
Fiore, L. D. et al. Department of Veterans Affairs Cooperative Studies Program Clinical Trial comparing combined warfarin and aspirin with aspirin alone in survivors of acute myocardial infarction: primary results of the CHAMP study. Circulation 105, 557–563 (2002).
Randomised double-blind trial of fixed low-dose warfarin with aspirin after myocardial infarction. Coumadin Aspirin Reinfarction Study (CARS) Investigators. Lancet 350, 389–396 (1997).
Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B. C. & Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nature Med. 8, 1175–1181 (2002). The first report of the development of a state-of-the-art confocal based imaging technique that enables three-dimensional real-time visualization of thrombus formation in vivo.
Ni, H. et al. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc. Natl Acad. Sci. USA 100, 2415–2419 (2003).
Patel, D. et al. Dynamics of GPIIb/IIIa-mediated platelet-platelet interactions in platelet adhesion/thrombus formation on collagen in vitro as revealed by videomicroscopy. Blood 101, 929–936 (2003).
Kulkarni, S. et al. A revised model of platelet aggregation. J. Clin. Invest. 105, 783–791 (2000).
Ni, H. et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J. Clin. Invest. 106, 385–392 (2000). This report highlights the existence of a third adhesive ligand promoting platelet aggregation and arterial thrombus formation in mice lacking both vWf and fibrinogen.
Denis, C. et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc. Natl Acad. Sci. USA 95, 9524–9529 (1998).
Tronik-Le Roux, D. et al. Thrombasthenic mice generated by replacement of the integrin α(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood 96, 1399–1408 (2000).
Smyth, S. S., Reis, E. D., Vaananen, H., Zhang, W. & Coller, B. S. Variable protection of β3-integrin-deficient mice from thrombosis initiated by different mechanisms. Blood 98, 1055–1062 (2001).
Hodivala-Dilke, K. M. et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 (1999).
Yeh, C. H., Chang, M. C., Peng, H. C. & Huang, T. F. Pharmacological characterization and antithrombotic effect of agkistin, a platelet glycoprotein Ib antagonist. Br. J. Pharmacol. 132, 843–850 (2001).
Wu, D., Meiring, M., Kotze, H. F., Deckmyn, H. & Cauwenberghs, N. Inhibition of platelet glycoprotein Ib, glycoprotein IIb/IIIa, or both by monoclonal antibodies prevents arterial thrombosis in baboons. Arterioscler. Thromb. Vasc. Biol. 22, 323–328 (2002).
Ware, J., Russell, S. & Ruggeri, Z. M. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc. Natl Acad. Sci. USA 97, 2803–2808 (2000).
Lopez, J. A., Andrews, R. K., Afshar-Kharghan, V. & Berndt, M. C. Bernard-Soulier syndrome. Blood 91, 4397–4418 (1998).
Ramakrishnan, V. et al. Increased thrombin responsiveness in platelets from mice lacking glycoprotein V. Proc. Natl Acad. Sci. USA 96, 13336–13341 (1999).
Moog, S. et al. Platelet glycoprotein V binds to collagen and participates in platelet adhesion and aggregation. Blood 98, 1038–1046 (2001).
Kahn, M. L. et al. Glycoprotein V-deficient platelets have undiminished thrombin responsiveness and do not exhibit a Bernard-Soulier phenotype. Blood 94, 4112–4121 (1999).
Massberg, S. et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 197, 41–49 (2003).
Kato, K. et al. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood 2003 May 8 [epub ahead of print].
Moroi, M., Jung, S. M., Okuma, M. & Shinmyozu, K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J. Clin. Invest. 84, 1440–1445 (1989).
Konishi, H. et al. Platelets activated by collagen through immunoreceptor tyrosine-based activation motif play pivotal role in initiation and generation of neointimal hyperplasia after vascular injury. Circulation 105, 912–916 (2002).
Nieswandt, B. et al. Glycoprotein VI but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 20, 2120–2130 (2001). This study demonstrates the importance of GPVI in promoting platelet adhesion and activation on collagen, and somewhat unexpectedly, the relatively normal haemostatic function of mouse platelets lacking all β1 integrins.
Kehrel, B. et al. Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen-induced aggregation and spontaneous loss of disorder. Blood 71, 1074–1078 (1988).
Nieuwenhuis, H. K., Akkerman, J. W., Houdijk, W. P. & Sixma, J. J. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature 318, 470–472 (1985).
Sadler, J. E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 67, 395–424 (1998).
Sadler, J. E. et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb. Haemost. 84, 160–174 (2000).
Suh, T. T. et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 9, 2020–2033 (1995).
Holmback, K., Danton, M. J., Suh, T. T., Daugherty, C. C. & Degen, J. L. Impaired platelet aggregation and sustained bleeding in mice lacking the fibrinogen motif bound by integrin αIIbβ3. EMBO J. 15, 5760–5771 (1996).
Myers, D., Jr. et al. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J. Surg. Res. 108, 212–221 (2002).
Falati, S. et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 197, 1585–1598 (2003).
Subramaniam, M. et al. Defects in hemostasis in P-selectin-deficient mice. Blood 87, 1238–1242 (1996).
Vollmar, B., Schmits, R., Kunz, D. & Menger, M. D. Lack of in vivo function of CD31 in vascular thrombosis. Thromb. Haemost. 85, 160–164 (2001).
Falati, S. et al. Opposing effects of P-selectin and PECAM–1 on arterial thrombus growth and stability in vivo. J. Thromb. Haemost. (Suppl. 1), AOC054 (2003).
Mahooti, S. et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am. J. Pathol. 157, 75–81 (2000).
Weiss, E. J., Hamilton, J. R., Lease, K. E. & Coughlin, S. R. Protection against thrombosis in mice lacking PAR3. Blood 100, 3240–3244 (2002).
Sambrano, G. R., Weiss, E. J., Zheng, Y. W., Huang, W. & Coughlin, S. R. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature 413, 1146–1148 (2001). The first demonstration of the importance of platelet thrombin receptors in normal haemostasis and thrombosis.
Fabre, J. E. et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nature Med. 5, 1199–1202 (1999). This paper, and reference 71, describe the first report of P2Y 1 receptor targeting in mouse platelets. These studies demonstrate an important role for this receptor in normal haemostasis and thrombosis. Importantly, the bleeding diathesis in these mice was less severe than that observed in mice lacking the P2Y 12 receptor.
Leon, C. et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J. Clin. Invest. 104, 1731–1737 (1999).
Leon, C. et al. Key role of the P2Y(1) receptor in tissue factor-induced thrombin-dependent acute thromboembolism: studies in P2Y(1)-knockout mice and mice treated with a P2Y(1) antagonist. Circulation 103, 718–723 (2001).
Foster, C. J. et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Invest. 107, 1591–1598 (2001).
Hollopeter, G. et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 (2001).
Hechler, B. et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J. Exp. Med. 198, 661–667 (2003).
Oury, C. et al. A natural dominant negative P2X1 receptor due to deletion of a single amino acid residue. J. Biol. Chem. 275, 22611–22614 (2000).
Thomas, D. W. et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Invest. 102, 1994–2001 (1998).
Hirata, T., Ushikubi, F., Kakizuka, A., Okuma, M. & Narumiya, S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J. Clin. Invest. 97, 949–956 (1996).
Hirata, T. et al. Arg60 to Leu mutation of the human thromboxane A2 receptor in a dominantly inherited bleeding disorder. J. Clin. Invest. 94, 1662–1667 (1994).
Ma, H. et al. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP(3). Circulation 104, 1176–1180 (2001).
Hirsch, E. et al. Resistance to thromboembolism in PI3Kγ-deficient mice. FASEB J. 15, 2019–2021 (2001).
Law, D. A. et al. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature 401, 808–811 (1999).
Abtahian, F. et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299, 247–251 (2003).
Clements, J. L. et al. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J. Clin. Invest. 103, 19–25 (1999).
Judd, B. A. et al. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through αIIbβ3 and collagen receptors. Proc. Natl Acad. Sci. USA 97, 12056–12061 (2000).
Wang, D. et al. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13, 25–35 (2000).
Mangin, P. et al. A PLCγ2-independent platelet collagen aggregation requiring functional association of GPVI and integrin α(2)β(1). FEBS Lett. 542, 53–59 (2003).
Johnson, E. N., Brass, L. F. & Funk, C. D. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc. Natl Acad. Sci. USA 95, 3100–3105 (1998).
Langenbach, R. et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83, 483–492 (1995).
Lagarde, M., Byron, P. A., Vargaftig, B. B. & Dechavanne, M. Impairment of platelet thromboxane A2 generation and of the platelet release reaction in two patients with congenital deficiency of platelet cyclo-oxygenase. Br. J. Haematol. 38, 251–266 (1978).
Malmsten, C., Hamberg, M., Svensson, J. & Samuelsson, B. Physiological role of an endoperoxide in human platelets: hemostatic defect due to platelet cyclo-oxygenase deficiency. Proc. Natl Acad. Sci. USA 72, 1446–1450 (1975).
Horellou, M. H. et al. Familial and constitutional bleeding disorder due to platelet cyclo-oxygenase deficiency. Am. J. Hematol. 14, 1–9 (1983).
Offermanns, S., Toombs, C. F., Hu, Y. H. & Simon, M. I. Defective platelet activation in G α(q)-deficient mice. Nature 389, 183–186 (1997). The first demonstration on the fundamental importance of G q and PLC-mediated phosphoinositide turnover in promoting the haemostatic function of platelets.
Kelleher, K. L., Matthaei, K. I. & Hendry, I. A. Targeted disruption of the mouse Gz-α gene: a role for Gz in platelet function? Thromb. Haemost. 85, 529–532 (2001).
Law, D. A. et al. Genetic and pharmacological analyses of Syk function in αIIbβ3 signaling in platelets. Blood 93, 2645–2652 (1999).
Massberg, S. et al. Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J. Exp. Med. 189, 1255–1264 (1999).
Angelillo-Scherrer, A. et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nature Med. 7, 215–221 (2001). Demonstrates an important role for Gas6 in amplifying platelet activation in response to multiple stimuli. Mice deficient in Gas6 demonstrate defective thrombus formation without prolongation of bleeding time.
Andre, P. et al. CD40L stabilizes arterial thrombi by a β3 integrin-dependent mechanism. Nature Med. 8, 247–252 (2002). This study demonstrates an important role for sCD40L in stabilizing platelet thrombus formation in vivo , through direct binding to integrin αIIbβ3.
Ruggeri, Z. M. von Willebrand factor. J. Clin. Invest. 99, 559–564 (1997).
George, J. N., Sadler, J. E. & Lammle, B. Platelets: thrombotic thrombocytopenic purpura. Hematology (Am. Soc. Hematol. Educ. Program) 315–334 (2002).
Ruggeri, Z. M. Old concepts and new developments in the study of platelet aggregation. J. Clin. Invest. 105, 699–701 (2000).
Savage, B., Almus-Jacobs, F. & Ruggeri, Z. M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 94, 657–666 (1998).
Savage, B., Saldivar, E. & Ruggeri, Z. M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84, 289–297 (1996). A seminal study highlighting the dynamic nature of platelet adhesion on a vWf substrate under physiologically relevant shear conditions.
Nievelstein, P. F., D'Alessio, P. A. & Sixma, J. J. Fibronectin in platelet adhesion to human collagen types I and III. Use of nonfibrillar and fibrillar collagen in flowing blood studies. Arteriosclerosis 8, 200–206 (1988).
Bonnefoy, A., Harsfalvi, J., Pfliegler, G., Fauvel-Lafeve, F. & Legrand, C. The subendothelium of the HMEC-1 cell line supports thrombus formation in the absence of von Willebrand factor and collagen types I, III and VI. Thromb. Haemost. 85, 552–559 (2001).
Beumer, S. et al. Platelet adhesion to fibronectin in flow: the importance of von Willebrand factor and glycoprotein Ib. Blood 86, 3452–3460 (1995).
Beumer, S., IJesseldijk, M. J., de Groot, P. G. & Sixma, J. J. Platelet adhesion to fibronectin in flow: dependence on surface concentration and shear rate, role of platelet membrane glycoproteins GP IIb/IIIa and VLA-5, and inhibition by heparin. Blood 84, 3724–3733 (1994).
Houdijk, W. P., Sakariassen, K. S., Nievelstein, P. F. & Sixma, J. J. Role of factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J. Clin. Invest. 75, 531–540 (1985).
Moroi, M. et al. Analysis of platelet adhesion to a collagen-coated surface under flow conditions: the involvement of glycoprotein VI in the platelet adhesion. Blood 88, 2081–2092 (1996).
Turitto, V. T., Weiss, H. J., Zimmerman, T. S. & Sussman, I. I. Factor VIII/von Willebrand factor in subendothelium mediates platelet adhesion. Blood 65, 823–831 (1985).
Weiss, H. J. et al. Fibrinogen-independent platelet adhesion and thrombus formation on subendothelium mediated by glycoprotein IIb–IIIa complex at high shear rate. J. Clin. Invest. 83, 288–297 (1989).
Ikeda, Y. et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J. Clin. Invest. 87, 1234–1240 (1991).
Ruggeri, Z. M., Dent, J. A. & Saldivar, E. Contribution of distinct adhesive interactions to platelet aggregation in flowing blood. Blood 94, 172–178 (1999).
Tsuji, S. et al. Real-time analysis of mural thrombus formation in various platelet aggregation disorders: distinct shear-dependent roles of platelet receptors and adhesive proteins under flow. Blood 94, 968–975 (1999).
Nieswandt, B. & Watson, S. P. Platelet collagen interaction: is GPVI the central receptor? Blood 2003 Mar 20 [epub ahead of print].
van Zanten, G. H. et al. Increased platelet deposition on atherosclerotic coronary arteries. J. Clin. Invest. 93, 615–632 (1994).
Gibbins, J. M., Okuma, M., Farndale, R., Barnes, M. & Watson, S. P. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 413, 255–259 (1997).
Tsuji, M., Ezumi, Y., Arai, M. & Takayama, H. A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J. Biol. Chem. 272, 23528–23531 (1997).
Nieswandt, B. et al. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chain. J. Biol. Chem. 275, 23998–24002 (2000).
Berlanga, O. et al. The Fc receptor γ-chain is necessary and sufficient to initiate signalling through glycoprotein VI in transfected cells by the snake C-type lectin, convulxin. Eur. J. Biochem. 269, 2951–2960 (2002).
Goto, S. et al. Involvement of glycoprotein VI in platelet thrombus formation on both collagen and von Willebrand factor surfaces under flow conditions. Circulation 106, 266–272 (2002).
Nieswandt, B. et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J. Exp. Med. 193, 459–469 (2001).
Kato, K. et al. Impaired thrombosis formation in a murine model of glycoprotein VI deficiency. J. Thromb. Haemost. (Suppl. 1), AOC147 (2003).
Jackson, S. P., Nesbitt, W. S. & Kulkarni, S. Signalling events underlying thromus formation. J. Thromb. Haemost. 1, 1602–1612 (2003).
Nieuwenhuis, H. K., Sakariassen, K. S., Houdijk, W. P., Nievelstein, P. F. & Sixma, J. J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood 68, 692–695 (1986).
Kroll, M. H. & Schafer, A. I. Biochemical mechanisms of platelet activation. Blood 74, 1181–1195 (1989).
Fressinaud, E., Sakariassen, K. S., Rothschild, C., Baumgartner, H. R. & Meyer, D. Shear rate-dependent impairment of thrombus growth on collagen in nonanticoagulated blood from patients with von Willebrand disease and hemophilia A. Blood 80, 988–994 (1992).
Inauen, W., Baumgartner, H. R., Bombeli, T., Haeberli, A. & Straub, P. W. Dose- and shear rate-dependent effects of heparin on thrombogenesis induced by rabbit aorta subendothelium exposed to flowing human blood. Arteriosclerosis 10, 607–615 (1990).
Gast, A., Tschopp, T. B. & Baumgartner, H. R. Thrombin plays a key role in late platelet thrombus growth and/or stability. Effect of a specific thrombin inhibitor on thrombogenesis induced by aortic subendothelium exposed to flowing rabbit blood. Arterioscler. Thromb. 14, 1466–1474 (1994).
Alevriadou, B. R. et al. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood 81, 1263–1276 (1993).
Barstad, R. M. et al. Reduced effect of aspirin on thrombus formation at high shear and disturbed laminar blood flow. Thromb. Haemost. 75, 827–832 (1996).
Roald, H. E. et al. Modulation of thrombotic responses in moderately stenosed arteries by cigarette smoking and aspirin ingestion. Arterioscler. Thromb. 14, 617–621 (1994).
Barstad, R. M., Roald, H. E., Cui, Y., Turitto, V. T. & Sakariassen, K. S. A perfusion chamber developed to investigate thrombus formation and shear profiles in flowing native human blood at the apex of well-defined stenoses. Arterioscler. Thromb. 14, 1984–1991 (1994).
Coughlin, S. R. How the protease thrombin talks to cells. Proc. Natl Acad. Sci. USA 96, 11023–11027 (1999).
Cattaneo, M. & Gachet, C. The platelet ADP receptors. Haematologica 86, 346–348 (2001).
Gachet, C. Platelet activation by ADP: the role of ADP antagonists. Ann. Med. 32 (Suppl. 1), 15–20 (2000).
Goldsack, N. R., Chambers, R. C., Dabbagh, K. & Laurent, G. J. Thrombin. Int. J. Biochem. Cell Biol. 30, 641–646 (1998).
Coughlin, S. R. & Camerer, E. PARticipation in inflammation. J. Clin. Invest. 111, 25–27 (2003).
Gachet, C. Identification, characterization, and inhibition of the platelet ADP receptors. Int. J. Hematol. 74, 375–381 (2001).
Kahn, M. L. et al. A dual thrombin receptor system for platelet activation. Nature 394, 690–694 (1998).
Rekhter, M. D. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc. Res. 41, 376–384 (1999).
Toschi, V. et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 95, 594–599 (1997).
Badimon, J. J. et al. Local inhibition of tissue factor reduces the thrombogenicity of disrupted human athersclerotic plaques. Effects of tissue factor pathway inhibitor on plaque thrombogenicity under flow conditions. Circulation 99, 1780–1787 (1999).
Siess, W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta 1582, 204–215 (2002).
Siess, W. et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl Acad. Sci. USA 96, 6931–6936 (1999).
Libby, P. Coronary artery injury and the biology of atherosclerosis: Inflammation, thrombosis and stabilization. Am. J. Cardiol. 86 (Suppl.), 3J–9J (2000).
Mallat, Z. et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques. A role for apoptosis in plaque thrombogenicity. Circulation 99, 348–353 (1999).
Trip, M. D., Cats, V. M., van Capelle, F. J. L. & Vreeken, J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N. Engl. J. Med. 322, 1549–1554 (1990).
Hirsh, J. Hyperactive platelets and complications of coronary artery disease. N. Engl. J. Med. 316, 1543–1544 (1987).
Roffi, M. et al. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 104, 2767–2771 (2001).
Lincoff, A. M. Important triad in cardiovascular medicine: diabetes, coronary intervention, and platelet glycoprotein IIb/IIIa receptor blockade. Circulation 107, 1556–1559 (2003).
Kjeldsen, S. E., Rostrup, M., Gjesdal, K. & Eide, I. The epinephrine-blood platelet connection with special reference to essential hypertension. Am. Heart J. 122, 330–336 (1991).
Hamet, P., Skuherska, R., Pang, S. C. & Tremblay, J. Abnormalities of platelet function in hypertension and diabetes. Hypertension 7, II135–II142 (1985).
Opper, C. et al. Increased number of high sensitive platelets in hypercholesterolemia, cardiovascular diseases, and after incubation with cholesterol. Atherosclerosis 277, 15225–15228 (1995).
Terres, W., Becker, P. & Rosenberg, A. Changes in cardiovascular risk profile during the cessation of smoking. Am. J. Med. 97, 242–249 (1994).
Terres, W., Weber, K., Kupper, W. & Bleifeld, W. Age, cardiovascular risk factors and coronary heart disease as determinants of platelet function in men. A multivariate approach. Thromb. Res. 62, 649–661 (1991).
Renaud, S. C., Beswick, A. D., Fehily, A. M., Sharp, D. S. & Elwood, P. C. Alcohol and platelet aggregation: the Caerphilly Prospective Heart Disease Study. Am. J. Clin. Nutr. 55, 1012–1017 (1992).
Mori, T. A., Beilin, L. J., Burke, V., Morris, J. & Ritchie, J. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 17, 279–286 (1997).
Santoso, S. Platelet polymorphisms in thrombotic disorders. Transfus. Clin. Biol. 8, 261–266 (2001).
Gonzalez-Conejero, R. et al. Polymorphisms of platelet membrane glycoprotein Ib associated with arterial thrombotic disease. Blood 92, 2771–2776 (1998).
Baker, R. I. et al. Platelet glycoprotein Ibα Kozak polymorphism is associated with an increased risk of ischemic stroke. Blood 98, 36–40 (2001).
Jilma-Stohlawetz, P. et al. Glycoprotein Ib polymorphisms influence platelet plug formation under high shear rates. Br. J. Haematol. 120, 652–655 (2003).
Burr, D., Doss, H., Cooke, G. E. & Goldschmidt-Clermont, P. J. A meta-analysis of studies on the association of the platelet PlA polymorphism of glycoprotein IIIa and risk of coronary heart disease. Stat. Med. 22, 1741–1760 (2003).
Kunicki, T. J. & Ruggeri, Z. M. Platelet collagen receptors and risk prediction in stroke and coronary artery disease. Circulation 104, 1451–1453 (2001).
Long, Q., Xu, X. Y., Ramnarine, K. V. & Hoskins, P. Numerical investigation of physiologically realistic pulsatile flow through arterial stenosis. J. Biomech. 34, 1229–1242 (2001).
Wootton, D. M. & Ku, D. N. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1, 299–329 (1999).
Deplano, V. & Siouffi, M. Experimental and numerical study of pulsatile flows through stenosis: wall shear stress analysis. J. Biomech. 32, 1081–1090 (1999).
Moritz, M. W., Reimers, R. C., Baker, R. K., Sutera, S. P. & Joist, J. H. Role of cytoplasmic and releasable ADP in platelet aggregation induced by laminar shear stress. J. Lab. Clin. Med. 101, 537–544 (1983).
Moake, J. L., Turner, N. A., Stathopoulos, N. A., Nolasco, L. & Hellums, J. D. Shear-induced platelet aggregation can be mediated by vWF released from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and is resistant to aspirin. Blood 71, 1366–1374 (1988).
Chow, T. W., Hellums, J. D., Moake, J. L. & Kroll, M. H. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood 80, 113–120 (1992).
Jen, C. J. & McIntire, L. V. Characteristics of shear-induced aggregation in whole blood. J. Lab. Clin. Med. 103, 115–124 (1984).
Konstantopoulos, K. et al. Flow cytometric studies of platelet responses to shear stress in whole blood. Biorheology 32, 73–93 (1995).
Hardwick, R. A., Hellums, J. D., Peterson, D. M., Moake, J. L. & Olson, J. D. The effect of PGI2 and theophylline on the response of platelets subjected to shear stress. Blood 58, 678–681 (1981).
Zambrowicz, B. P. & Sands, A. T. Knockouts model the 100 best-selling drugs — will they model the next 100? Nature Rev. Drug Discov. 2, 38–51 (2003).
Gurevitz, O. et al. S-nitrosoderivative of a recombinant fragment of von Willebrand factor (S-nitroso-AR545C) inhibits thrombus formation in guinea pig carotid artery thrombosis model. Thromb. Haemost. 84, 912–917 (2000).
Gurevitz, O. et al. Recombinant von Willebrand factor fragment AR545C inhibits platelet aggregation and enhances thrombolysis with rtPA in a rabbit thrombosis model. Arterioscler. Thromb. Vasc. Biol. 18, 200–207 (1998).
Kageyama, S. et al. Anti-thrombotic effects and bleeding risk of AJvW-2, a monoclonal antibody against human von Willebrand factor. Br. J. Pharmacol. 122, 165–171 (1997).
Azzam, K., Cisse-Thiam, M. & Drouet, L. The antithrombotic effect of aurin tricarboxylic acid in the guinea pig is not solely due to its interaction with the von Willebrand factor-GPIb axis. Thromb. Haemost. 75, 203–210 (1996).
Chang, M. C., Lin, H. K., Peng, H. C. & Huang, T. F. Antithrombotic effect of crotalin, a platelet membrane glycoprotein Ib antagonist from venom of Crotalus atrox. Blood 91, 1582–1589 (1998).
Kageyama, S., Yamamoto, H., Nakazawa, H. & Yoshimoto, R. Anti-human vWF monoclonal antibody, AJvW-2 Fab, inhibits repetitive coronary artery thrombosis without bleeding time prolongation in dogs. Thromb. Res. 101, 395–404 (2001).
Marcus, A. J. Thrombosis and inflammation as multicellular processes: significance of cell-cell interactions. Semin. Hematol. 31, 261–269 (1994).
Simon, D. I. et al. Platelet glycoprotein ibα is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Exp. Med. 192, 193–204 (2000).
Romo, G. M. et al. The glycoprotein Ib–IX–V complex is a platelet counterreceptor for P-selectin. J. Exp. Med. 190, 803–814 (1999).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Cauwenberghs, N. et al. Antithrombotic effect of platelet glycoprotein Ib-blocking monoclonal antibody Fab fragments in nonhuman primates. Arterioscler. Thromb. Vasc. Biol. 20, 1347–1353 (2000).
Cadroy, Y. et al. Relative antithrombotic effects of monoclonal antibodies targeting different platelet glycoprotein-adhesive molecule interactions in nonhuman primates. Blood 83, 3218–3224 (1994).
Takahashi, R., Sekine, N. & Nakatake, T. Influence of monoclonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation. Blood 93, 1951–198 (1999).
Alimardani, G., Guichard, J., Fichelson, S. & Cramer, E. M. Pathogenic effects of anti-glycoprotein Ib antibodies on megakaryocytes and platelets. Thromb. Haemost. 88, 1039–1046 (2002).
Wu, D. et al. Inhibition of the von Willebrand (VWF)-collagen interaction by an antihuman VWF monoclonal antibody results in abolition of in vivo arterial platelet thrombus formation in baboons. Blood 99, 3623–3628 (2002).
Ojio, S. et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans: coronary angiographic findings within 1 week before the onset of infarction. Circulation 102, 2063–2069 (2000). An important study providing angiographic evidence that the majority of patients presenting with an acute myocardial infarction have plaque rupture and superimposed non-occlusive thrombus formation, up to one week before hospital presentation.
Smith, J. B. & Willis, A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nature New Biol. 231, 235–237 (1971).
Inhibition of vascular and platelet prostaglandin synthesis by aspirin. N. Engl. J. Med. 309, 670–672 (1983).
Randomized trial of ridogrel, a combined thromboxane A2 synthase inhibitor and thromboxane A2/prostaglandin endoperoxide receptor antagonist, versus aspirin as adjunct to thrombolysis in patients with acute myocardial infarction. The Ridogrel Versus Aspirin Patency Trial (RAPT). Circulation 89, 588–595 (1994).
Michaux, C. et al. Terbogrel, a dual-acting agent for thromboxane receptor antagonism and thromboxane synthase inhibition. Acta Crystallogr. C 56, 1265–1266 (2000).
Soyka, R., Guth, B. D., Weisenberger, H. M., Luger, P. & Muller, T. H. Guanidine derivatives as combined thromboxane A2 receptor antagonists and synthase inhibitors. J. Med. Chem. 42, 1235–1249 (1999).
Langleben, D. et al. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am. Heart J. 143, E4 (2002).
Mangin, P. et al. Signaling role for PLCγ 2 in platelet glycoprotein Ibα calcium flux and cytoskeletal reorganization involvement of a pathway distinct from FcR γ-chain and cγ RIIA. J. Biol. Chem. 2003 Jun 17 [epub ahead of print].
Andre, P., Nannizzi-Alaimo, L., Prasad, S. K. & Phillips, D. R. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation 106, 896–899 (2002).
Mangano, D. T. Aspirin and mortality from coronary bypass surgery. N. Engl. J. Med. 347, 1309–1317 (2002).
Grotta, J. C. & Alexandrov, A. V. Preventing stroke: is preventing microemboli enough? Circulation 103, 2321–2322 (2001).
Acknowledgements
The authors wish to thank Drs Zaverio Ruggeri, Christian Gachet, Hatem Salem and Francois Lanza, as well as other members of the authors' laboratory for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
FURTHER INFORMATION
Glossary
- BLEEDING TIME
-
The time taken for cessation of bleeding from a standardized injury, which is used to test for platelet abnormalities (acquired and congenital) and also for von Willebrand's disease.
- THERAPEUTIC WINDOW
-
The dose (or concentration) range between the minimum effective dose (concentration) and the minimum toxic dose (concentration).
- RHEOLOGICAL ENVIRONMENT
-
The blood-flow characteristics operating within defined areas of the vasculature.
- THROMBOTIC THROMBOCYTOPAENIC PURPURA
-
A rare microvascular thrombotic disorder associated with low platelet count, fever, neurological and renal impairment and micro-angiopathic haemolytic anaemia.
- DIATHESIS
-
A permanent (hereditary or acquired) condition that predisposes the affected individual to certain diseases.
- THROMBOCYTOPAENIA
-
A decrease in platelet count, defined in humans as less than 150 × 109 per l, which results in the potential for increased bleeding.
- PHARMACOPHORE
-
The ensemble of steric and electronic features that are necessary to ensure optimal interactions with a specific biological target structure and to trigger (or to block) its biological response.
- CAROTID ENDARTERECTOMY
-
A surgical procedure that removes atherosclerotic plaque from the walls of the carotid arteries in an attempt to reduce the risk of stroke or transient ischaemic attack.
- PERCUTANEOUS CORONARY INTERVENTION
-
A group of techniques that can relieve coronary artery narrowing. The techniques include balloon angioplasty, laser angioplasty and implantation of intracoronary stents and other catheter devices.
Rights and permissions
About this article
Cite this article
Jackson, S., Schoenwaelder, S. Antiplatelet therapy: in search of the 'magic bullet'. Nat Rev Drug Discov 2, 775–789 (2003). https://doi.org/10.1038/nrd1198
Issue Date:
DOI: https://doi.org/10.1038/nrd1198
This article is cited by
-
Platelet CFTR inhibition enhances arterial thrombosis via increasing intracellular Cl− concentration and activation of SGK1 signaling pathway
Acta Pharmacologica Sinica (2022)
-
Design, synthesis and evaluation of new chromone-derived aminophosphonates as potential acetylcholinesterase inhibitor
Molecular Diversity (2021)
-
In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbα
Scientific Reports (2021)
-
The chimeric monoclonal antibody MHCSZ-123 against human von Willebrand factor A3 domain inhibits high-shear arterial thrombosis in a Rhesus monkey model
Journal of Hematology & Oncology (2017)
-
A novel ruthenium (II)-derived organometallic compound, TQ-6, potently inhibits platelet aggregation: Ex vivo and in vivo studies
Scientific Reports (2017)