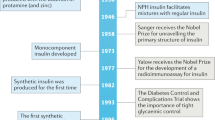
Key Points
-
Despite the introduction of insulin therapy, the pharmacokinetics of conventional insulin preparations given subcutaneously makes it difficult to replicate the normal pattern of nutrient-related and basal insulin secretion.
-
Realizing the dream of administering insulin orally has met with little, if any, success, despite the use of many strategies to overcome the barriers to absorption that are presented by the gastrointestinal tract. Administering insulin through the mucosa of the mouth is a potentially attractive option. However, the buccal and sublingual mucosae present special problems for insulin delivery due to the combined effects of the relatively thick, multilayered buccal barrier and the constant flow of saliva. Although the skin is easily accessible and has a large surface area, it is relatively impermeable to large hydrophilic polypeptides, such as insulin. The nasal mucosa is another potentially attractive route, but local barriers to absorption exist in the form of an active mucociliary transport mechanism and an enzymatically active, low-permeability, low-pH nasal epithelium.
-
The respiratory tree offers the largest available surface area for drug delivery, and provides an attractive option for the systemic delivery of drugs and polypeptide hormones.
-
Modern inhaler devices produce a polydisperse aerosol, and include a wide variety of nebulizers, pressurized metered-dose inhalers (pMDIs) and dry-powder inhalers (DPIs). These devices are limited by their dependence on the inspiratory flow rate, and hence patient technique, and are subject to large inter- and intra-subject variability. DPI devices are further limited by powder hygroscopicity, which reduces the respirable fraction.
-
The development of the intrapulmonary route has benefited from an improved understanding of the importance of aerosol particle size, inspiratory flow rate and inhaled volume. More research is continuing to ensure intrapulmonary delivery becomes a clinical reality. Specifically, methods of estimating bioavailability, biopotency and safety need to be standardized, and precision of dosing, dose adjustment and reproducibility remain key features. Assessing cost benefit is an integral part of the evaluation process.
-
Several other treatment options might be possible, including pancreas transplantation and islet-cell allotransplantation, and on the far horizon are exciting opportunities afforded by advancements in cell biology and genetics, some or all of which might provide the final opportunity for insulin independence.
-
This new millennium promises a revolutionary change in the delivery of insulin, which cannot come too soon for the billions of sufferers who are reliant on subcutaneous administration. Cautious optimism based on scientific rigour is the only way forward.
Abstract
Since the introduction of insulin therapy 80 years ago, the lives of millions of patients with diabetes have been saved, prolonged and immeasurably improved. However, restoring normal glucose levels in diabetic patients through administering insulin by subcutaneous injection has proved virtually impossible. The consequences for patients are serious complications, including diabetic retinopathy and nephropathy, which tend to result from persistent hyperglycaemia. Maximizing glucose control in diabetic patients requires several daily injections. In an effort to reduce this burden, alternative and less-intrusive routes for the administration of insulin are being explored.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banting, F. G., Best, C. H., Collip, J. B., Campbell, J. J. R. & Fletcher, A. A. Pancreatic extracts in the treatment of diabetes mellitus: preliminary report. Can. Med. Assoc. J. 12, 141–146 (1922).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Eng. J. Med. 329, 977–986 (1993).
Lauritzen, T., Faber, O. K. & Binder, C. Variation in 125I-insulin absorption and blood glucose concentration. Diabetologia 17, 291–295 (1979).
Binder, C., Lauritzen, T., Faber, O. & Pramming, S. Insulin pharmacokinetics. Diabetes Care 7, 188–199 (1984).
Galloway, T. A. et al. Factors influencing the absorption versus insulin concentration and blood glucose responses after injections of regular insulin in various insulin mixtures. Diabetes Care 4, 366–376 (1981).
Barnett, A. H. & Owens, D. R. Insulin analogues. Lancet 349, 47–51 (1997).
Owens, D. R., Zinman, B. & Bolli, G. Insulins today and beyond. Lancet 358, 739–746 (2001).An up-to-date review of insulin preparations with specific reference to the new rapid- and long-acting insulin analogues.
Slama, G., Hautecouverture, M., Assan, R. & Tchobroutsky, G. One to five days of continuous intravenous insulin infusion on seven diabetic patients. Diabetes 23, 732–738 (1974).
Pickup, J. C., White, M. C., Keen, H., Parsons, J. A. & Alberti, K. G. M. M. Long-term continuous subcutaneous insulin infusion in diabetes at home. Lancet 2, 870–873 (1979).
Selam, J.-L. in Transplantation and Changing Management of Organ Failure (ed. Cochat, P.) 131–138 (Kluwer Academic, Dordrecht, 2000).
Pickup, J. & Keen, H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 25, 593–598 (2002).
Buchwald, H. et al. A totally implantable drug infusion device: laboratory and clinical experiences using a model with single flow rate and new design for modulated insulin infusion. Diabetes Care 3, 351–358 (1980).
Irsigler, K. et al. Long-term continuous intraperitoneal insulin infusion with an implanted remote-controlled insulin infusion device. Diabetes 30, 1072–1075 (1981).
Gin, H. et al. Clinical evaluation of a newly designed compliant side port catheter for an insulin implantable pump: The EVADIAC experience. Diabetes Care 24, 175 (2001).
Shimoda, S. et al. Closed-loop subcutaneous insulin infusion algorithm with a short-acting insulin analog for long-term clinical application of a wearable artificial pancreas. Front. Med. Biol. Eng. 8, 197–211 (1997).
Shichiri, M., Sakakida, M., Nishida, K. & Shimoda, S. Enhanced, simplified glucose sensors: long-term clinical application of wearable artificial endocrine pancreas. Artificial Organs 22, 32–42 (1998).
Brange, J. & Langkjaer, L. in Protein Delivery: Physical Systems (eds Sanders, L. M. and Hendren, R. W.) 343–412 (Plenum, New York, 1997).An excellent and detailed review of insulin formulations and different routes of delivery that relate predominantly to animal studies.
Chetty, D. J. & Chien, Y. W. Novel methods of insulin delivery: an update. Crit. Rev. Ther. Drug Carrier Syst. 15, 629–670 (1998).
Berger, M. in Frontiers in Insulin Pharmacology (eds Berger, M. & Gries, F.) 144–148 (Plenum, Stuttgart, 1993).
Carino, G. P. & Mathiowitz, E. Oral insulin delivery. Adv. Drug Deliv. Rev. 35, 249–257 (1999).
Damgé, C. in Biotechnology of Insulin Therapy (ed. Pickup, J.) 97–112 (Blackwell Scientific, Oxford, 1991).
Patel, H. M. & Ryman, B. E. Orally administered liposomally entrapped insulin. Biochem. Soc. Trans. 5, 1739–1741 (1977).
Langer, R. Drug delivery and targeting. Nature 392, 5–10 (1998).An important review of the different methodologies that are used for drug delivery and targeting. Current and future opportunities are discussed.
Mathiowitz, E. et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature 386, 410–414 (1997).
Milstein, S. J. et al. Partially unfolded efficiently penetrated cell membranes — implications for oral drug delivery. J. Control. Release 53, 259–267 (1998).
Still, J. G. Development of oral insulin: progress and current status. Diabetes/Metabolism: Research and Reviews 18, S29–S37 (2002).
Crane, C. W., Path, M. C. & Luntz, G. R. W. N. Absorption of insulin from the human small intestine. Diabetes 17, 625–627 (1968).
Murlin, J. R. Effectiveness of peroral insulin in human diabetes. J. Clin. Invest. 19, 709–722 (1940).
Patel, H. M. et al. Intrajejunal absorption of liposomally entrapped insulin in normal man. Biochem. Soc. Trans. 6, 784–785 (1978).
Earle, M. P. Experimental use of oral insulin. Isr. J. Med. Soc. 8, 899–900 (1972).
Galloway, J. A. & Root, M.A. New forms of insulin. Diabetes 21 (Suppl. 2), 637–648 (1972).
Gwinup, G., Elias, A. N. & Domurat, E. S. Insulin and C-peptide levels following oral administration of insulin in intestinal enzyme protected capsules. Gen. Pharmacol. 22, 243–246 (1991).
Nagai, T. & Machida, Y. Mucosal adhesive dosage forms. Pharm. Int. 6, 196–200 (1985).
Aungst, B. J. & Rogers, N. J. Site dependence of absorption-promoting actions of laureth-9, Na salicylate, Na2EDTA, and aprotinin on rectal, nasal, and buccal insulin delivery. Pharm. Res. 5, 305–308 (1988).
Veuillez, F., Kalia, Y. N., Jacques, Y., Deshusses, J. & Buri, P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 51, 93–109 (2001).This is an expansive review of the many factors and strategies that are being used to enhance buccal absorption of peptides and other therapeutic agents.
Al-Walli, N. S. D. Sublingual human insulin for hyperglycaemia in type 1 diabetes. J. Pak. Med. Assoc. 49, 167–169 (1999).
Schwartz, S. & Modi, P. Pharmacodynamics of oral insulin in healthy volunteers. Diabetologia 43 (Suppl. 1), A202 (2000).
Modi, P. & Mihic, M. A comparison of oral insulin versus subcutaneous injection in type 2 diabetic patients. Diabetologia 43 (Suppl. 1), A203 (2000).
Caldwell, L., Nishihata, T., Rytting, J. H. & Higuchi, T. Lymphatic uptake of water-soluble drugs after rectal administration. J. Pharm. Pharmacol. 34, 520–542 (1982).
Matsuda, H. & Arima, H. Cyclodextrins in transdermal and rectal delivery. Adv. Drug Deliv. Rev. 36, 81–99 (1999).
Yun, M.-O., Choi, H.-G., Jung, J.-H. & Kim, C.-K. Development of a thermo-reversible liquid suppository with bioavailabilty enhancement. Int. J. Pharm. 189, 137–145 (1999).
Yamasaki, Y. et al. The effectiveness of rectal administration of insulin suppository on normal and diabetic subjects. Diabetes Care 4, 454–458 (1981).
Hildebrant, R., Lius, A., Lotz, U. & Schliack, V. Effect of insulin suppositories in type 1 diabetic patients. Exp. Clin. Endocrinol. 873, 168–172 (1984).
Raz, I., Bar-on, H., Kidron, M. & Ziv, E. Rectal administration of insulin. Isr. J. Med. Sci. 20, 173–175 (1984).
Stephen, R. L., Petelenz, T. J. & Jacobsen, S. C. Potential novel methods for insulin administration: I. Iontophoresis. Biomed. Biochim. Acta 43, 553–558 (1984).
Sage, B. H. Jr. Protein Delivery — Physical Systems (eds Saunders, L. M. & Hendren, R. W.) 319–341 (Plenum Publishing, New York, 1997).
Siddiqui, O., Shi, W. M. & Chien, Y. W. Transdermal iontophoretic delivery of insulin for blood glucose control in diabetic rabbits. Proc. Int. Symp. Control. Res. Bioact. Mater. 14, 174 (1987).
Langkjaer, L., Brange, J., Grodsky, G. M. & Guy, R. H. Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pre-treatment. J. Control. Release 51, 47–56 (1998).
Tachibana, K. Transdermal delivery of insulin to alloxan-diabetic rabbits by ultrasound exposure. Pharm. Res. 9, 952–4 (1992).
Mitragotri, S., Blankschtein, D. & Langer, R. Ultrasound-mediated transdermal protein delivery. Science 269, 850–853 (1995).
Cevc, G. Transfersomes, liposomes and other liquid suspensions on the skin: permeation enhancement vesicle penetration and transdermal drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 13, 257–388 (1996).
Gizurarson, S. & Bechgaard, E. Intranasal administration of insulin to humans. Diabetes Res. Clin. Pract. 12, 71–84 (1991).
Hinchcliffe, M. & Illum, L. Intranasal insulin delivery and therapy. Adv. Drug Deliv. Rev. 35, 199–234 (1999).This review deals with the intranasal delivery of drugs with consideration to the structure and function of the nasal cavity.
Frauman, A. G., Jerums, G. & Louis, W. J. Effects of intranasal insulin in non-obese type 2 diabetics. Diabetes Res. Clin. Pract. 3, 197–202 (1987).
Drejer, K. et al. Pharmacokinetics of intranasally administered insulin with phospholipids as absorption enhancers. Diabetologia 53 (Suppl.1), A61 (1990).
Moses, A. C. Insulin administered intranasally as an insulin-bile salt aerosol — effectiveness and reproducibility in normal and diabetic subjects. Diabetes 32, 1040–1041 (1983).
Hirai, S., Ikenaga, T. & Matzuzawa, T. Nasal absorption of insulin in dogs. Diabetes 27, 296–299 (1978).
Duchateau, G. S. M. J. E. et al. Bile salts and intranasal drug absorption. Int. J. Pharmacy 31, 193–199 (1986).
Lee, W. A. et al. Histological studies of insulin absorption across the nasal mucosa in the presence of sodium taurodihydrofusidate (STDHF). Proc. Int. Symp. Control. Release Biochem. Mater. 15, 77 (1988).
Drejer, K. et al. Intranasal insulin administration of insulin with phospholipid as absorption enhancer: pharmacokinetics in normal subjects. Diabetic Med. 9, 335–340 (1992).
Pontiroli, A. E. et al. Insulin given intranasally induces hypoglycaemia in normal and diabetic subjects. Br. Med. J. 284, 303–306 (1982).
Bruce, D. G., Chishom, D. J., Storlien, L. H., Borkman, M. & Kraegen, E. W. Meal-time intranasal insulin delivery in type 2 diabetes. Diabetic Med. 8, 366–370 (1991).
Coates, P. A. Intranasal insulin: the effect of three dose regimens on postprandial glycaemic profiles in type 2 diabetic subjects. Diabetic Med. 12, 235–239 (1995).
Salzman, R. et al. Intranasal aerosalized insulin. Mixed-meal studies and long-term use in type 1 diabetes. N. Engl. J. Med. 312, 1078–1084 (1985).
Lassmann-Vague, V., Thiers, D., Vialettes, B. & Vague, P. Preprandial intranasal insulin. Lancet 13, 367–368 (1988).
Hilsted, J. et al. Intranasal insulin therapy: the clinical realities. Diabetologia 38, 680–684 (1995).
Frauman, A. G., Cooper, M. E., Parsons, B. J., Jerums, G. & Louis, W. J. Long-term use of intranasal insulin in insulin-dependant diabetic patients. Diabetes Care 10, 573–578 (1987).
Lelej-Bennis, D. et al. Efficacy and tolerance of intranasal insulin administered during 4 months in severely hyperglycaemic type 2 diabetic patients with oral drug failure: a cross over study. Diabetic Med. 18, 614–618 (2001).
Lelej-Bennis, D. et al. Six month administration of gelified intranasal insulin in type 1 diabetic patients under multiple injections: efficacy versus subcutaneous injections and local tolerance. Diabetes Metab. 27, 372–377 (2001).
Holinger, M. A. Respiratory Pharmacology and Toxicology (W. B. Saunders, Philadelphia, 1985).
Weibel, E. R. in Handbook of Physiology (eds Ferm, W. O. & Rahn, I. I.) 284–307 (Am. Physiol. Soc., Washington DC, 1964).
Byron, P. R. Determinants of drug and polypeptide bioavailabilty from aerosols delivered to the lung. Adv. Drug Deliv. Rev. 5, 107–132 (1990).
Patton, J. S., Bukar, J. & Nagarajan, S. Inhaled insulin. Adv. Drug Deliv. Rev. 35, 235–247 (1999).This review addresses the many determinants of drug and peptide bioavailability, from aerosol delivery to the lung.
Patton, J. S. & Platz, R. M, Pulmonary delivery of peptides and proteins for systemic action. Adv. Drug Deliv. Rev. 8, 179–196 (1992).A discussion of the many barriers to the absorption of drugs and peptides to the lung, and the mechanisms of absorption.
Von Heubner, W., de Jongh, S. E. & Laquer, E. Uber inhalation von insulin. Klin. Wochenschrift 51, 2342–2343 (1924).
Gansslen, M. Uber inhalation von insulin. Klin. Wochenschrift 4, 71 (1925).
Wigley, F. M. et al. Insulin across respiratory mucosae by aerosol delivery. Diabetes 20, 552–556 (1971).
Byron, P. R. & Patton, J. S. Drug delivery via the respiratory tract. J. Aerosol Med. 7, 49–75 (1994).
Schultz, H. Mechanisms and factors affecting intrapulmonary particle deposition: implications for efficient inhalation therapies. Pharm. Sci. Technol. 1, 336–344 (1998).
Farr, S. J. et al. Pulmonary insulin administration using the AERx™ system: physiological and physiochemical factors influencing insulin effectiveness in healthy fasting subjects. Diabetes Technol. Therapeutics 2, 185–197 (2000).
Katz, I. M., Schroeter, J.D. & Martonen, T. B. Factors affecting the deposition of aerosolized insulin. Diabetes Technol. Therapeutics 3, 387–397 (2001).
Martonen, T. B. Mathematical model for the selective deposition of inhaled pharmaceuticals. J. Pharm. Sci. 82, 1191–1199 (1993).
Martonen, T. B. et al. Human lung morphology models for particle deposition studies. Inhal. Toxicol. 12, 109–121 (2000).
Wollmer, P. & Evander, E. Biphasic pulmonary clearance of 99mTc-DPTA in smokers. Clin. Physiol. 14, 547–559 (1994).
Bradvik, I. One year follow-up of lung clearance of 99m c-diethylene traimine penta-acetic acid and diseases activity in sarcodisis. Vasc. Diffuse Lung Dis. 17, 281–287 (2000).
Jones, J. G., Royston, D. & Minty, B. D. Changes in alveolar–capillary barrier function in animals and humans. Am. Rev. Respir. Dis. 127, S51–S59 (1983).
Minty, B. D., Royston, D., Jones, J. G. & Hulands, G. H. The effect of nicotine on pulmonary epithelial permeability in man. Chest 86, 72–74 (1984).
Meignam, M. Exercise increases the lung clearance of inhaled technetium-99m DPTA. J. Nuclear Med. 27, 274–280 (1986).
Schmekel, B., Borgstrom, L. & Wollmer, P. Exercise increases the rate of pulmonary absorption of inhaled terbulatin. Chest 1, 742–745 (1992).
Fink, J. B. Metered-dose inhalers, dry powder inhalers, and transitions. Resp. Care 456, 623–635 (2000).
Smith, K. J., Chan. H. K. & Brown, K. F. Influence of flow rate on aerosol particle size distributions from pressurised and breath-actuated inhalers. J. Aerosol Med. 11, 231–245 (1998).
Corne, J., Gillespie, D., Roberts, D. & Younes, M. Effect of inspiratory flow rate in intubated ventilated patients. Am. J. Respir. Crit. Care Med. 156, 304–308 (1997).
Borgstom, L., Bengtsson, T., Derom, E. & Pauwels, R. Variability in lung deposition of inhaled drug, within and between asthmatic patients, with a pMDI and dry powder inhaler, Turbuhaler. Int. J. Pharm. 193, 227–230 (2000).
Maggi, L., Bruni, R. & Conte, U. Influence of the moisture on the performance of a new dry powder inhaler. Int. J. Pharm. 177, 83–91 (1999).
Heise, T. Time–action profile of an inhaled insulin preparation in comparison to insulin lispro and regular insulin. Diabetes 39 (Suppl. 1), A10 (2000).
Gelfand, R. A., Schwartz, S. L., Horton, M., Law, C. G. & Pun, E. F. Pharmacological reproducibility of inhaled human insulin in patients with type 2 diabetes mellitus. Diabetologia 43, 773 (2000).
Skyler, J. S. et al. Efficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study. Lancet 357, 331–335 (2001).A pivotal clinical study that tested the hypothesis of pulmonary delivery of insulin in comparison to subcutaneous insulin for meal-related requirements in patients with type 1 diabetes. The formulation of insulin was a dry powder, which was packaged into a single-dose blister that was placed into a delivery device.
Quattrin, T. Efficacy and safety of inhaled insulin (Exubera) compared to conventional subcutaneous insulin therapy in patients with type 1 diabetes: results of a six month randomised trial. Am. Assoc. Clin. Endocrinol. Annu. Meet. Clin. Congr. Syllabus, 205 (2002).
Cefalu, W. T. et al. Inhaled human insulin treatment in patients with type 2 diabetes mellitus. Ann. Int. Med. 134, 203–207 (2001).
Farr, S. J. et al. Comparison of in vitro and in vivo efficiencies of a novel unit-dose aerosol generator and a pressurised metered dose inhaler. Int. J. Pharm. 198, 63–70 (2000).
Kipnes, M. Pharmacokinetics and pharmacodynamics of pulmonary insulin delivered via the AERx diabetes management system in type 1 diabetics. Diabetologia 43 (Suppl. 1), A201 (2000).
McElduff, A. et al. Comparison of the pharmacokinetics and pharmacodynamics of subcutaneous and inhaled insulin lispro in healthy fasted volunteers. Diabetes 47, 413 (1998).
Jendle, J. et al. Pharmacokinetics of pulmonary insulin in healthy smokers and non-smokers. Diabetologia 44 (Suppl. 1), 816 (2001).
Henry, R. et al. Pulmonary delivery of insulin using the AERx™ insulin diabetes management system in healthy and asthmatic subjects. Diabetologia 44 (Suppl. 1), 9 (2001).
Brunner, G. et al. Dose–response relation of liquid aerosol inhaled insulin in type I diabetic patients. Diabetologia 44, 305–308 (2001).An important study that used aerosolized liquid insulin in patients with type 1 diabetes. It clearly showed dose–response relationships in both bioavailability and activity of the inhaled insulin.
Fishman, R. S., Guinta, D., Chambers, F., Quintana, R. & Shapiro, D. A. Insulin administration via the Aerodose™ inhaler: comparison to subcutaneously injected insulin. Diabetes 49 (Suppl. 1), 38 (2000).
Heinemann, L. et al. Impact of particle size and aerosolisation time on the metabolic effect of an inhaled insulin aerosol. Diabetologia 44 (Suppl. 1), 10 (2001).
Edwards, D. A., Ben-Jerbria, A., Eskew, M. L. & Langer, R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J. Appl. Physiol. 85, 379–385 (1998).
Edwards, D. A. et al. Large porous particles for pulmonary drug delivery. Science 276, 1868–1871 (1997).
Vanbever, R., Ben-jebria, A., Mintzes, J. D., Langer, R. & Edwards, D. A. Sustained release of insulin from insoluble inhaled particles. Drug Dev. Res. 48, 178–185 (1999).
Hrkach, J. AIR insulin: complete diabetes therapy via inhalation of fact-acting and slow-acting dry powder aerosols. Diabetes 49 (Suppl. 1), 37 (2000).
Steiner, S. et al. Technosphere™/insulin: bioavailibilty and pharmacokinetic properties in healthy volunteers. Diabetologia 43 (Suppl. 1), 771 (2000).
Perera, A. D. et al. Reproducibility of inhaled and subcutaneous insulin in type 2 diabetic patients. Diabetologia 44 (Suppl. 1), 815 (2001).
Kohler, D. Nicht radioaktives verfahren zur messung der lungenpermeabilitat: inhalation von insulin. Atemwegs Lungenkrankh 13, 230–232 (1987).
Schmekel, B., Borgstrom, L. & Wollmer, P. Difference in pulmonary absorption of inhaled terbutaine in healthy smokers and non-smokers. Thorax 46, 225–228 (1991).
Wise, S. D., Sathirakul, K., Yeo, K. P., Chien, J. Y. & Aftring, R. P. Smoking increases the bioavailibility of inhaled insulin, but relative insulin resistance ameliorates differences in action. Diabetologia 44 (Suppl. 1), 12 (2001).
Weiss, S. R. et al. Adjunctive therapy with inhaled human insulin in type 2 diabetic patients failing oral agents: a multicenter phase II trial. Diabetes 48 (Suppl. 1), 48 (1999)
Chan, N. N. et al. Inhaled insulin in type 1 diabetes. Lancet 357, 1979–1980 (2001).
Baker, E. H. & Phillips, B. J. Inhaled insulin in type 1 diabetes. Lancet 357, 1979–1980 (2001).
Acknowledgements
I would like to thank S. Griffiths and L. Edwards for their help in the preparation of this manuscript, A. Shaw for the illustrations and G. Dodson for the insulin R6 hexamer structure.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
Medscape DrugInfo
OMIM
Glossary
- HYPERGLYCAEMIA
-
An abnormally high level of glucose in the blood. It occurs when the body does not have enough insulin (insulin deficiency) or cannot use the insulin it does have (insulin resistance) to metabolize glucose.
- NEPHROPATHY
-
A degenerative kidney disease that is associated with long-standing diabetes. Diabetic nephropathy is caused by damage to the glomerular capillaries. Features of the disorder include proteinuria, hypertension and progressive impairment of kidney function, which could, ultimately, lead to kidney failure.
- RETINOPATHY
-
A degenerative disorder of the retina that is associated with long-standing diabetes. Diabetic retinopathy is one of the main causes of blindness. In an initial phase of the disorder, damage to retinal blood vessels causes leakage of fluid into the eye. Subsequently, new retinal vessels might form that, due to their fragile nature, could cause haemorrhage and subsequent blindness.
- PHARMACOKINETICS
-
The study of the absorption, distribution, metabolism, excretion and interactions of a drug.
- IONTOPHORESIS
-
The movement of ions under the influence of an applied electric field. The technique can be used to improve the absorption of drugs across the skin.
- SONOPHORESIS
-
A method that is applied to increase transdermal drug delivery by the use of a high-frequency ultrasound system.
- AMPHIPHILIC
-
One end of the molecule is hydrophilic and the other is hydrophobic.
- BIOAVAILABILITY
-
The fraction or percentage of an administered drug or other substance that becomes available to the target tissue after administration.
- EXCIPIENT
-
A largely inert substance that is added to a drug formulation to improve administration or absorption.
- TYPE 2 DIABETES
-
A late-onset form of diabetes in which the pancreas produces insufficient insulin (insulin deficiency) or cannot use insulin (insulin resistance). Various treatments are used to control the condition, including dietary restriction, exercise, oral hypoglycaemic agents or injected insulin.
- TYPE 1 DIABETES
-
An early-onset form of diabetes in which the β-cells of the pancreas produce little or no insulin. The condition is controlled with daily subcutaneous insulin injections or insulin that is delivered by either an external pump into the subcutaneous tissue or intraperitoneally.
- MUCOCILIARY
-
Human airways are lined with a ciliary membrane. These cilia are ∼5 μm in length, and as the cilia beat, they engage with, and propel, a thin overlying layer of mucus.
- HYGROSCOPICITY
-
The tendency of a drug or other substance to absorb or discharge moisture according to environmental conditions.
- NEBULIZER
-
A device that uses pressurized air to turn a liquid medication into a fine mist for inhalation.
- HbA1C
-
A modified form of haemoglobin that contains covalently bound glucose molecules. HbA1c levels are used to monitor long-term glycaemic control in diabetic patients. The HbA1c level reflects the average blood-glucose level over the previous four months. The HbA1c level in normal subjects is less than 7%. In poorly controlled diabetic subjects, levels above 12% could be seen.
- SCINTIGRAPHY
-
A diagnostic test in which a two-dimensional picture of the internal structure of an organ is obtained. Radiolabelled chemicals are injected into the blood and are taken up by specific tissues. X-rays are used to produce an image of the organ.
- PHAGOCYTOSIS
-
The process of the engulfment and destruction of extracellular materials by phagocytic cells, such as macrophages and neutrophils.
- ALLOTRANSPLANTATION
-
The transfer of cells, tissues and/or whole organs from one individual to another within the same species.
Rights and permissions
About this article
Cite this article
Owens, D. New horizons — alternative routes for insulin therapy. Nat Rev Drug Discov 1, 529–540 (2002). https://doi.org/10.1038/nrd836
Issue Date:
DOI: https://doi.org/10.1038/nrd836
This article is cited by
-
Nanomedicine’s transformative impact on anti-diabetic drug discovery: an appraisal
Journal of Nanoparticle Research (2023)
-
Insulin: evolution of insulin formulations and their application in clinical practice over 100 years
Acta Diabetologica (2022)
-
3D structure prediction of VAPC1 and identification of dual natural inhibitors for VPAC1 and EGFR
Journal of Bioenergetics and Biomembranes (2019)
-
Glucose-responsive oral insulin delivery for postprandial glycemic regulation
Nano Research (2019)
-
Novel route of insulin delivery using an implant-mediated drug delivery system
Drug Delivery and Translational Research (2017)