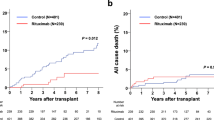
Abstract
The success of cardiac transplantation is largely attributable to the development of effective immunosuppressive regimens. The introduction of calcineurin inhibitors was pivotal in reducing the frequency of acute rejection and improving early survival. Newer agents, including mycophenolate mofetil (MMF) and proliferation-signal inhibitors, have shown promise in further reducing acute-rejection rates and, notably, reducing the frequency of cardiac allograft vasculopathy, which limits long-term graft survival. The introduction of first-year intravascular ultrasonography results as a surrogate marker for outcome after cardiac transplantation has helped assessment of the efficacy of immunosuppressive medications. Proliferation-signal inhibitors and MMF were shown by this imaging method to reduce cardiac allograft vasculopathy. The combination of these drugs, in tandem with the weaning of patients off calcineurin inhibitors, has been shown to reverse calcineurin-inhibitor-related nephrotoxic effects. A randomized trial that compared three of the more common immunosuppressive regimens suggested that tacrolimus and MMF are associated with a reduction in the frequency of rejection episodes that require treatment and have the fewest adverse effects. Finally, the use of statins has brought added benefit to immunosuppressive regimens by improving outcomes after cardiac transplantation, reportedly because of an immunomodulatory property. Promising newer immunosuppressive agents await clinical trials. This review presents an overview of the emerging data on immunosuppressive therapy for cardiac transplantation.
Key Points
-
Cardiac allograft rejection (and subsequent infection) is the major cause of death in the first year after heart transplantation
-
Newer immunosuppressive agents, such as mycophenolate mofetil and proliferation-signal inhibitors, have significantly reduced first-year cardiac allograft rejection rates
-
Induction therapy with cytolytic agents and interleukin-2-receptor antagonists is commonly used, but its efficacy and safety have yet to be confirmed
-
Cardiac allograft vasculopathy is primarily immune-mediated and can be reduced by immunosuppressive agents, such as mycophenolate mofetil, sirolimus, and everolimus
-
Statins, in addition to their potent cholesterol-lowering effects, seem to have notable immunomodulating effects in heart-transplant patients
-
Various novel immunosuppressive agents seem promising, but results from clinical, randomized trials will be needed to confirm their safety and efficacy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Caves PK et al. (1973) Results of 54 cardiac transplants. Surgery 74: 307–314
Auphan N et al. (1995) Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270: 286–290
Scheinman RI et al. (1995) Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 270: 283–286
Olivari MT et al. (1995) Triple-drug immunosuppression with steroid discontinuation by six months after heart transplantation. J Heart Lung Transplant 14: 127–135
Kobashigawa JA et al. (1995) Corticosteroid weaning late after heart transplantation: relation to HLA-DR mismatching and long-term metabolic benefits. J Heart Lung Transplant 14: 963–967
Opelz G et al. (2005) Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant 5: 720–728
Borel JF et al. (1977) Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology 32: 1017–1025
Oyer P et al. (1983) Cyclosporine in cardiac transplantation: a 2½ year follow-up. Transplant Proc 15 (Suppl 1–2): S2546–S2581
Cheung A and Menkis AH (1998) Cyclosporine heart transplantation. Transplant Proc 30: 1881–1884
Cooney GF et al. (1998) Comparative bioavailability of Neoral and Sandimmune in cardiac transplant recipients over 1 year. Transplant Proc 30: 1892–1894
Eisen HJ et al. (2001) Safety tolerability and efficacy of cyclosporine microemulsion in heart transplant recipients: a randomized multicenter double-blind comparison with the oil-based formulation of cyclosporine—results at 24 months after transplantation. Transplantation 71: 70–78
Carrier M et al. (1997) Comparison of Neoral and Sandimmune cyclosporine for induction of immunosuppression after heart transplantation. Can J Cardiol 13: 469–473
Maccherini M et al. (1998) Neoral versus Sandimmun: clinical impact and modification of immunosuppressive therapy in cardiac transplantation. Transplant Proc 30: 1904–1905
Yonan NA et al. (1998) Long-term safety and efficacy of Neoral in heart transplantation. Transplant Proc 30: 1906–1909
Cantarovich M et al. (1999) Clinical benefit of neoral dose monitoring with cyclosporine 2-hr post-dose levels compared with trough levels in stable heart transplant patients. Transplantation 68: 1839–1842
Taylor DO et al. (1999) A randomized multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J Heart Lung Transplant 18: 336–345
Reichart B et al. (2001) European multicenter tacrolimus heart pilot study: three year follow-up. J Heart Lung Transplant 20: 249–250
Armitage JM et al. (1992) Clinical trial of FK 506 immunosuppression in adult cardiac transplantation. Ann Thorac Surg 54: 205–210
Mentzer RM Jr et al. (1998) Tacrolimus as a rescue immunosuppressant after heart and lung transplantation. The US Multicenter FK506 Study Group. Transplantation 65: 109–113
Meiser BM et al. (1997) TACROLIMUS: a superior agent to OKT3 for treating cases of persistent rejection after intrathoracic transplantation. J Heart Lung Transplant 16: 795–800
Taylor DO et al. (2001) Suggested guidelines for the use of tacrolimus in cardiac transplant recipients. J Heart Lung Transplant 20: 734–738
Kobashigawa JA et al. (2006) Five-year results of a randomized, single-center study of tacrolimus versus microemulsion cyclosporine in heart transplant patients. J Heart Lung Transplant [doi: 10.1016/j.healun.2005.11.452]
Edwards L and Taylor DO (2005) Does the choice of immunosuppressive agent impact the development of renal insufficiency following heart transplantation? Am J Transplant 5 (Suppl 11): 249
Baran DA et al. (2002) Current practices: immounosuppression. Curr Opin Cardiol 17: 165–170
Delgado DH et al. (2005) Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant 24: 166–169
Cantarovich M et al. (2002) Anti-CD25 monoclonal antibody coverage allows for calcineurin inhibitor “holiday” in solid organ transplant patients with acute renal dysfunction. Transplantation 73: 1169–1172
Hershberger RE et al. (2005) Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med 352: 2705–2713
Opelz G and Dohler B (2004) Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 4: 222–230
Kobashigawa J et al. (1998) A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 66: 507–515
Radovancevic B and Vrtovec B (2003) Sirolimus therapy in cardiac transplantation. Transplant Proc 35 (Suppl): S171–S176
Keogh A et al. (2004) Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation 110: 2694–2700
Mancini D et al. (2003) Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation 108: 48–53
King-Biggs MB et al. (2003) Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 75: 1437–1443
Dean PG et al (2004) Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 77: 1555–1561
Eisen HJ et al. (2003) Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med 349: 847–858
Stallone G et al. (2005) Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 352: 1317–1323
Kobashigawa J et al. 12 month report of a 3 arm multicenter comparision of tacrolimus MMF or Tac/Sirolimus and steroids vs cyclosporine microemulsion MMF and steroids in de novo cardiac transplant recipients. Am J Transplant, in press
Angermann CE et al. (2004) Reduction of cyclosporine after introduction of mycophenolate mofetil improves chronic renal dysfunction in heart transplant recipients—the IMPROVED multi-centre study. Eur Heart J 25: 1626–1634
Groetzner J et al. (2004) Mycophenolate mofetil and sirolimus as calcineurin inhibitor-free immunosuppression for late cardiac-transplant recipients with chronic renal failure. Transplantation 77: 568–574
Kobashigawa JA et al. (1995) Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 333: 621–627
Wenke K et al. (2003) Simvastatin initiated early after heart transplantation: 8-year prospective experience. Circulation 107: 93–97
Kobashigawa JA et al. (2005) Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. J Heart Lung Transplant [doi: 10.1016/j.healun.2005.02.009]
Kwak B et al. (2000) Statins as a newly recognized type of immunomodulator. Nat Med 6: 1399–1402
Kobashigawa JA et al. (2004) Statins in solid organ transplantation: is there an immunosuppressive effect? Am J Transplant 4: 1013–1018
Downward J et al. (1990) Stimulation of p21ras upon T-cell activation. Nature 346: 719–723
Wei X et al. (2000) Perillyl alcohol inhibits TCR-mediated [Ca2+]i signaling, alters cell shape and motility, and induces apoptosis in T lymphocytes. Cell Immunol 201: 6–13
Maggard MA et al. (1999) Inhibition of transplant vasculopathy by pravastatin is associated with attenuated host allo-antibody response. Transpl Proc 1–2: 728–729
Rose EA et al. (1989) Humoral immune responses after cardiac transplantation correlation with fatal rejection and graft atherosclerosis. Surgery 106: 203–207
Park MH et al. (1998) Anti-donor HLA antibodies associated with allograft arteriopathy detected using intravascular ultrasound. Transplantation 65 (Suppl): S26
Maggard MA et al. (1998) Effects of pravastatin on chronic rejection of rat cardiac allografts. Transplantation 65: 149–155
Calne R et al. (1999) Campath IH allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation 68: 1613–1616
Knechtle SJ et al. (2003) Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant 3: 722–730
Vincenti F et al. (2005) Costimulation blockade with belatacept in renal transplantation. N Engl J Med 353: 770–781
Pierson RN III et al. (1999) Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation 68: 1800–1805
Kobashigawa J et al. Further analysis of the intravascular ultrasound data from the randomised mycopenolate mofetil (MMF) trial in heart transplant recipients. Am J Transplant, in press
Kobashigawa JA et al. (2005) Multicenter intravascular ultrasound (IVUS) validation study among heart transplant recipients: outcomes after 5 years. J Am Coll Cardiol 45: 1532–1537
Halloran PF and Gourishankar S (2001) Principles and overview of immunosuppression. In Primer on Transplantation, edn 2, 87–98 (Eds Norman DJ and Turka LA) Mount Laurel: American Society of Transplantation
Borie DC et al. (2005) Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation 79: 791–801
Higuchi T et al. (2005) Prevention of acute lung allograft rejection in rat by the janus kinase 3 inhibitor, tyrphostin AG490. J Heart Lung Transplant 24: 1557–1564
Williams JW et al. (2002) Experiences with leflunomide in solid organ transplantation. Transplantation 73: 358–366
Hardinger KL et al. (2002) Prospective, pilot, open-label, short-term study of conversion to leflunomide reverses chronic renal allograft dysfunction. Am J Transplant 2: 867–871
Tedesco-Silva H et al. (2005) FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 79: 1553–1560
Vincenti F et al. (2001) A phase I/II trial of anti-CD11a monoclonal antibody in renal transplantation. Am J Transplant 1 (Suppl): S276
Kirk A et al. (2001) Preliminary results of the use of humanized anti-CD154 in human renal transplantation. Am J Transplant 1 (Suppl): S191
Yun JJ et al. (2004) Combined blockade of the chemokine receptors CCR1 and CCR5 attenuates chronic rejection. Circulation 109: 932–937
Si MS et al. (2003) Farnesyltransferase inhibition: a novel method of immunomodulation. Int Immunopharmacol 3: 475–483
Acknowledgements
We thank Pamela Almeda and Grace Wu for preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JA Kobashigawa and J Patel have received research grants for clinical trials from Novartis, Roche, Astellas Pharma. JA Kobashigawa has served on advisory boards for Novartis, Roche and Astellas, and has received honoraria for lectures from these companies.
Rights and permissions
About this article
Cite this article
Kobashigawa, J., Patel, J. Immunosuppression for heart transplantation: where are we now?. Nat Rev Cardiol 3, 203–212 (2006). https://doi.org/10.1038/ncpcardio0510
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0510
This article is cited by
-
Regulation of T cell alloimmunity by PI3Kγ and PI3Kδ
Nature Communications (2017)
-
Cellular and Functional Imaging of Cardiac Transplant Rejection
Current Cardiovascular Imaging Reports (2011)
-
Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart
Nature Medicine (2008)