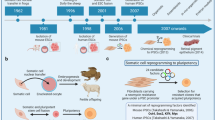
Abstract
Pluripotent cells, such as embryonic stem cells, are invaluable tools for research and can potentially serve as a source of cell- and tissue-replacement therapy. Rejection after transplantation of cells and tissue derived from embryonic stem cells is a significant obstacle to their clinical use. Recently, human somatic cells have been reprogrammed directly to pluripotency by ectopic expression of four transcription factors (Oct4, Sox2, Klf4 and Myc) to yield induced pluripotent stem (iPS) cells. Human iPS cells are a potential source of patient-specific pluripotent stem cells that would bypass immune rejection. iPS cells can also be used to study diseases for which there are no adequate human in vitro or animal models. In this protocol, we describe how to establish primary human fibroblasts lines and how to derive iPS cells by retroviral transduction of reprogramming factors. Overall, it takes 2 months to complete reprogramming human primary fibroblasts starting from biopsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
04 September 2008
In Figure 2a of the PDF version of this article initially published online, five labels (OCT4, SOX2, MYC, KLF4 and βACT) were omitted from the left side of the figure. The error has been corrected in the PDF version of the article.
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Park, I.H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
Lowry, W.E. et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA 105, 2883–2888 (2008).
Takahashi, K., Okita, K., Nakagawa, M. & Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2, 3081–3089 (2007).
Tiscornia, G., Singer, O. & Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 1, 241–245 (2006).
Lerou, P.H. et al. Derivation and maintenance of human embryonic stem cells. Nat. Protoc. 3, 923–933 (2008).
Tian, X. & Kaufman, D.S. Hematopoietic development of human embryonic stem cells in culture. Methods Mol. Biol. 290, 149–162 (2005).
Acknowledgements
This work was made possible through the generosity and vision of Jonathan and Patti Kraft and private funds donated to the Children's Hospital Boston. G.Q.D. is supported by grants from the National Institutes of Health and the NIH Director's Pioneer Award, and the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Park, IH., Lerou, P., Zhao, R. et al. Generation of human-induced pluripotent stem cells. Nat Protoc 3, 1180–1186 (2008). https://doi.org/10.1038/nprot.2008.92
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.92
This article is cited by
-
The lethal and sterile doses of gamma radiation on the museums pest, varied carpet beetle, Anthrenus verbasci (Coleoptera: Dermestidae)
Scientific Reports (2023)
-
Exosomes induce neurogenesis of pluripotent P19 cells
Stem Cell Reviews and Reports (2023)
-
Mispatterning and interneuron deficit in Tourette Syndrome basal ganglia organoids
Molecular Psychiatry (2022)
-
Routine culture and study of adult human brain cells from neurosurgical specimens
Nature Protocols (2022)
-
A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair
Signal Transduction and Targeted Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.