Abstract
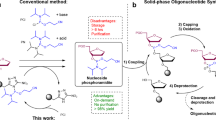
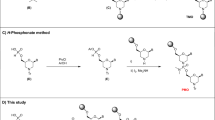
A protocol for the rapid, automated synthesis of oligospermine–oligonucleotide sequences is described. To this end, a protected spermine phosphoramidite derivative was synthesized in six steps from spermine and used as the fifth synthon in an oligonucleotide synthesizer. Parameters were optimized to reach greater than 95% coupling yields. Cationic oligonucleotides show enhanced hybridization and strand invasion properties, and hence are an alternative to conventional oligonucleotides for molecular biology, diagnostic and potential therapeutic applications. A multi-gram-scale synthesis of the spermine phosphoramidite allowing several hundred coupling steps takes 2–3 weeks. Oligonucleotide synthesis and purification takes approximately 3 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Manoharan, M. RNA interference and chemically modified small interfering RNAs. Curr. Opin. Chem. Biol. 8, 570–579 (2004).
Phillips, M.I. Ed. Methods in Enzymology, 313: Antisense Technology Part A & B (Academic, San Diego, California, 2000).
De Mesmaeker, A., Haener, R., Martin, P. & Moser, H.E. Antisense oligonucleotides. Acc. Chem. Res. 28, 366–374 (1995).
Lemaitre, M., Bayard, B. & Lebleu, B. Specific antiviral activity of a poly(l-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc. Natl. Acad. Sci. USA. 84, 648–652 (1987).
Wei, Z., Tung, C.H., Zhu, T. & Stein, S. Synthesis of oligoarginine-oligonucleotide conjugates and oligoarginine-bridged oligonucleotide pairs. Bioconjug. Chem. 5, 468–474 (1994).
Corey, D.R. 48000-fold Acceleration of hybridization by chemically modified oligonucleotides. J. Am. Chem. Soc. 117, 9373–9374 (1995).
Pitie, M. & Meunier, B. Cleavage of double-stranded DNA by manganese tris(methylpyridiniumyl)porphyrin linked to 3′-spermine oligonucleotides. J. Biol. Inorg. Chem. 1, 239–246 (1996).
Wei, Z. et al. Hybridization properties of oligodeoxynucleotide pairs bridged by polyarginine peptides. Nucleic Acids Res. 24, 655–61 (1996).
Harrison, J.G. & Balasubramanian, S. Synthesis and hybridization analysis of a small library of peptide-oligonucleotide conjugates. Nucleic Acids Res. 26, 3136–3145 (1998).
Stetsenko, D.A., Arzumanov, A.A., Korshun, V.A. & Gait, M.J. Peptide conjugates of oligonucleotides as enhanced antisense agents. Mol. Biol. (Translation of Molekulyarnaya Biologiya (Moscow)) 34, 852–859 (2000).
Zatsepin, T.S. et al. Synthesis of peptide-oligonucleotide conjugates with single and multiple peptides attached to 2′-aldehydes through thiazolidine, oxime, and hydrazine linkages. Bioconjug. Chem. 13, 822–830 (2002).
Chen, C.-P. et al. A concise method for the preparation of peptide and arginine-rich peptide-conjugated antisense oligonucleotide. Bioconjug. Chem. 14, 532–538 (2003).
Fraley, A.W. et al. Cationic oligonucleotide-peptide conjugates with aggregating properties enter efficiently into cells while maintaining hybridization properties and enzymatic recognition. J. Am. Chem. Soc. 128, 10763–10771 (2006).
Pons, B., Kotera, M., Zuber, G. & Behr, J.-P. Online synthesis of diblock cationic oligonucleotides for enhanced hybridization to their complementary sequence. Chembiochem 7, 1173–1176 (2006).
Bergeron, R.J. et al. Polyamine analogue antidiarrheals: a structure-activity study. J. Med. Chem. 44, 232–244 (2001).
Eckstein, F. (ed.) Oligonucleotides and Analogs: A Practical Approach (Oxford University Press, Oxford, New York, 1991).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Behr is a minor shareholder of a company that licensed this technology.
Rights and permissions
About this article
Cite this article
Voirin, E., Behr, JP. & Kotera, M. Versatile synthesis of oligodeoxyribonucleotide–oligospermine conjugates. Nat Protoc 2, 1360–1367 (2007). https://doi.org/10.1038/nprot.2007.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.177
This article is cited by
-
Globin mRNA reduction for whole-blood transcriptome sequencing
Scientific Reports (2016)
-
Zip nucleic acid: a new reliable method to increase the melting temperature of real-time PCR probes
Journal of Diabetes & Metabolic Disorders (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.