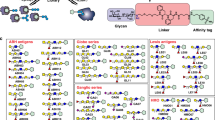
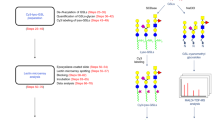
Abstract
Frontal affinity chromatography using fluorescence detection (FAC-FD) is a versatile technique for the precise determination of dissociation constants (Kd) between glycan-binding proteins (lectins) and fluorescent-labeled glycans. A series of glycan-containing solutions is applied to a lectin-immobilized column, and the elution profile of each glycan (termed the 'elution front', V) is compared with that (V0) for an appropriate control. Here we describe our standard protocol using an automated FAC system (FAC-1), consisting of two isocratic pumps, an autosampler, a column oven and two miniature columns connected to a fluorescence detector. Analysis time for 100 sugar–protein interactions is ∼10 h, using as little as 2.5 pmol of pyridylaminated (PA) oligosaccharide per analysis. Using FAC-FD, we have so far obtained quantitative interaction data of >100 lectins for >100 PA oligosaccharides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Crocker, P.R., Paulson, J.C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Robinson, M.J., Sancho, D., Slack, E.C., LeibundGut-Landmann, S. & Reis e Sousa, C. Myeloid C-type lectins in innate immunity. Nat. Immunol. 7, 1258–1265 (2006).
van Vliet, S.J., Dunnen, J.D., Gringhuis, S.I., Geijtenbeek, T.B. & van Kooyk, Y. Innate signaling and regulation of Dendritic cell immunity. Curr. Opin. Immunol. 19, 435–440 (2007).
Sharon, N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 282, 2753–2764 (2007).
Sharon, N. & Lis, H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14, 53R–62R (2004).
Hirabayashi, J. et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 (2002).
Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 101, 17033–17038 (2004).
Kuno, A. et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat. Methods 2, 851–856 (2005).
Hirabayashi, J., Hayama, K., Kaji, H., Isobe, T. & Kasai, K. Affinity capturing and gene assignment of soluble glycoproteins produced by the nematode Caenorhabditis elegans. J. Biochem. (Tokyo) 132, 103–114 (2002).
Hirabayashi, J. Lectin-based structural glycomics: glycoproteomics and glycan profiling. Glycoconj. J. 21, 35–40 (2004).
Knibbs, R.N., Osborne, S.E., Glick, G.D. & Goldstein, I.J. Binding determinants of the sialic acid-specific lectin from the slug Limax flavus. J. Biol. Chem. 268, 18524–18531 (1993).
Blixt, O., Collins, B.E., van den Nieuwenhof, I.M., Crocker, P.R. & Paulson, J.C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278, 31007–31019 (2003).
Collins, B.E. et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J. Immunol. 177, 2994–3003 (2006).
Yamaji, T., Teranishi, T., Alphey, M.S., Crocker, P.R. & Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 277, 6324–6332 (2002).
Mehta, P., Cummings, R.D. & McEver, R.P. Affinity and kinetic analysis of P-selectin binding to P-selectin glycoprotein ligand-1. J. Biol. Chem. 273, 32506–32513 (1998).
Adler, P. et al. High affinity binding of the Entamoeba histolytica lectin to polyvalent N-acetylgalactosaminides. J. Biol. Chem. 270, 5164–5171 (1995).
Tateno, H. & Goldstein, I.J. Partial identification of carbohydrate-binding sites of a Galalpha1,3Galbeta1,4GlcNAc-specific lectin from the mushroom Marasmius oreades by site-directed mutagenesis. Arch. Biochem. Biophys. 427, 101–109 (2004).
Winter, H.C., Oscarson, S., Slattegard, R., Tian, M. & Goldstein, I.J. Banana lectin is unique in its recognition of the reducing unit of 3-O-beta-glucosyl/mannosyl disaccharides: a calorimetric study. Glycobiology 15, 1043–1050 (2005).
Dam, T.K. et al. Binding studies of alpha-GaLNAc specific lectins to the alpha-GaLNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. J. Biol. Chem. (2007)(Epub ahead of print).
Dam, T.K. & Brewer, C.F. Multivalent protein-carbohydrate interactions: isothermal titration microcalorimetry studies. Methods Enzymol. 379, 107–128 (2004).
Brewer, C.F. Thermodynamic binding studies of galectin-1, -3 and -7. Glycoconj. J. 19, 459–465 (2004).
Tateno, H., Winter, H.C. & Goldstein, I.J. Cloning, expression in Escherichia coli and characterization of the recombinant Neu5Acalpha2,6Galbeta1,4GlcNAc-specific high-affinity lectin and its mutants from the mushroom. Polyporus squamosus. Biochem. J. 382, 667–675 (2004).
Powell, L.D., Jain, R.K., Matta, K.L., Sabesan, S. & : Varki, A. Characterization of sialyloligosaccharide binding by recombinant soluble and native cell-associated CD22. Evidence for a minimal structural recognition motif and the potential importance of multisite binding. J. Biol. Chem. 270, 7523–7532 (1995).
Shimura, K. & Kasai, K. Analysis of lectin-carbohydrate interactions by capillary affinophoresis. Methods Enzymol. 362, 398–417 (2003).
Shimura, K., Arata, Y., Uchiyama, N., Hirabayashi, J. & Kasai, K. Determination of the affinity constants of recombinant human galectin-1 and -3 for simple saccharides by capillary affinophoresis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 768, 199–210 (2002).
Mo, H., Winter, H.C. & Goldstein, I.J. Purification and characterization of a Neu5Acalpha2-6Galbeta1-4Glc/GlcNAc-specific lectin from the fruiting body of the polypore mushroom Polyporus squamosus. J. Biol. Chem. 275, 10623–10629 (2000).
Tateno, H., Winter, H.C., Petryniak, J. & Goldstein, I.J. Purification, characterization, molecular cloning, and expression of novel members of jacalin-related lectins from rhizomes of the true fern Phlebodium aureum (L) J. Smith (Polypodiaceae). J. Biol. Chem. 278, 10891–10899 (2003).
Goldstein, I.J. et al. Carbohydrate binding properties of banana (Musa acuminata) lectin II. Binding of laminaribiose oligosaccharides and beta-glucans containing beta1,6-glucosyl end groups. Eur. J. Biochem. 268, 2616–2619 (2001).
Mo, H. et al. Carbohydrate binding properties of banana (Musa acuminata) lectin I. Novel recognition of internal alpha1,3-linked glucosyl residues. Eur. J. Biochem. 268, 2609–2615 (2001).
Ohyama, Y., Kasai, K., Nomoto, H. & Inoue, Y. Frontal affinity chromatography of ovalbumin glycoasparagines on a concanavalin A-sepharose column. A quantitative study of the binding specificity of the lectin. J. Biol. Chem. 260, 6882–6887 (1985).
Oda, Y., Kasai, K. & Ishii, S. Studies on the specific interaction of concanavalin A and saccharides by affinity chromatography. Application of quantitative affinity chromatography to a multivalent system. J. Biochem. (Tokyo) 89, 285–296 (1981).
Kasai, K. & Ishii, S. Quantitative analysis of affinity chromatography of trypsin. A new technique for investigation of protein-ligand interaction. J. Biochem. (Tokyo) 77, 261–264 (1975).
Hirabayashi, J., Arata, Y. & Kasai, K. Frontal affinity chromatography as a tool for elucidation of sugar recognition properties of lectins. Methods Enzymol. 362, 353–368 (2003).
Hirabayashi, J., Arata, Y. & Kasai, K. Reinforcement of frontal affinity chromatography for effective analysis of lectin-oligosaccharide interactions. J. Chromatogr. A 890, 261–271 (2000).
Arata, Y., Hirabayashi, J. & Kasai, K.I. Application of reinforced frontal affinity chromatography and advanced processing procedure to the study of the binding property of a Caenorhabditis elegans galectin. J. Chromatogr. A 905, 337–343 (2001).
Nakamura, S., Yagi, F., Totani, K., Ito, Y. & Hirabayashi, J. Comparative analysis of carbohydrate-binding properties of two tandem repeat-type Jacalin-related lectins, Castanea crenata agglutinin and Cycas revoluta leaf lectin. FEBS J. 272, 2784–2799 (2005).
Nakamura-Tsuruta, S. et al. Comparative analysis by frontal affinity chromatography of oligosaccharide specificity of GlcNAc-binding lectins, Griffonia simplicifolia lectin-II (GSL-II) and Boletopsis leucomelas lectin (BLL). J. Biochem. (Tokyo) 140, 285–291 (2006).
Arata, Y., Hirabayashi, J. & : Kasai, K. The two lectin domains of the tandem-repeat 32-kDa galectin of the nematode Caenorhabditis elegans have different binding properties. Studies with recombinant protein. J. Biochem. (Tokyo) 121, 1002–1009 (1997).
Dam, T.K., Roy, R., Page, D. & Brewer, C.F. Negative cooperativity associated with binding of multivalent carbohydrates to lectins. Thermodynamic analysis of the 'multivalency effect'. Biochemistry 41, 1351–1358 (2002).
Dam, T.K., Roy, R., Page, D. & Brewer, C.F. Thermodynamic binding parameters of individual epitopes of multivalent carbohydrates to concanavalin a as determined by 'reverse' isothermal titration microcalorimetry. Biochemistry 41, 1359–1363 (2002).
Takahashi, N., Nakagawa, H., Fujikawa, K., Kawamura, Y. & Tomiya, N. Three-dimensional elution mapping of pyridylaminated N-linked neutral and sialyl oligosaccharides. Anal. Biochem. 226, 139–146 (1995).
Acknowledgements
The authors thank Junko Kominami (J-Oil Mills), Noboru Uchiyama, Yusuke Osaka (Shimadzu Co.) and all current and past members of the Hirabayashi Lab for their various contributions to the development and establishment of the FAC protocol; J-Oil Mills for providing GSL-II. This work was supported by New Energy and Industrial Technology Development Organization (NEDO) in Japan under the Ministry of Economy, Trade, and Industry (METI).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Data
Screening and analysis of GSL-II (PDF 3789 kb)
Rights and permissions
About this article
Cite this article
Tateno, H., Nakamura-Tsuruta, S. & Hirabayashi, J. Frontal affinity chromatography: sugar–protein interactions. Nat Protoc 2, 2529–2537 (2007). https://doi.org/10.1038/nprot.2007.357
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.357
This article is cited by
-
Discovery of a lectin domain that regulates enzyme activity in mouse N-acetylglucosaminyltransferase-IVa (MGAT4A)
Communications Biology (2022)
-
The trimeric solution structure and fucose-binding mechanism of the core fucosylation-specific lectin PhoSL
Scientific Reports (2018)
-
New platform for simple and rapid protein-based affinity reactions
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.