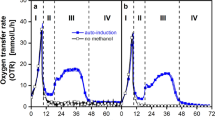
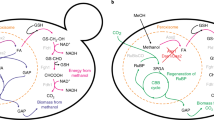
Abstract
This protocol is applicable to recombinant protein expression by small-scale fermentation using the Pichia pastoris expression system. P. pastoris has the capacity to produce large quantities of protein with eukaryotic processing. Expression is controlled by a methanol-inducible promoter, which allows a biomass-generation phase before protein production is initiated. The target protein is secreted directly into a protein-free mineral salt medium, and is relatively easy to purify. The protocol is readily interfaced with expanded bed adsorption for immediate capture and purification of recombinant protein. The setting up of the bioreactor plus the fermentation itself takes 1 wk. Making the master and user seed lots takes ∼2 wk for each individual clone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cregg, J.M., Cereghino, J.L., Shi, J. & Higgins, D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 16, 23–52 (2000).
Daly, R. & Hearn, M.T. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 18, 119–138 (2005).
Macauley-Patrick, S., Fazenda, M.L., McNeil, B. & Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 22, 249–270 (2005).
Baez, J., Olsen, D. & Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 69, 245–252 (2005).
Zhang, W., Bevins, M.A., Plantz, B.A., Smith, L.A. & Meagher, M.M. Modeling Pichia pastoris growth on methanol and optimizing the production of a recombinant protein, the heavy-chain fragment C of botulinum neurotoxin, serotype A. Biotechnol. Bioeng. 70, 1–8 (2000).
Tolner, B., Smith, L., Begent, R.H.J. & Chester, K.A. Expanded bed adsorption immobilized metal affinity chromatography. Nat. Protocols (in press).
Lee, Y.C. Structural and interaction studies of an anti-carcinoembryonic antigen single-chain Fv antibody: application to molecular design for cancer targeting. Ph.D. Thesis, University of London, (2003).
Sainz-Pastor, N. et al. Deglycosylation to obtain stable and homogeneous Pichia pastoris-expressed N-A1 domains of carcinoembryonic antigen. Int. J. Biol. Macromol. (in press)
Sharma, S.K. et al. Sustained tumor regression of human colorectal cancer xenografts using a multifunctional mannosylated fusion protein in antibody-directed enzyme prodrug therapy. Clin. Cancer Res. 11, 814–825 (2005).
Francis, R.J. et al. Radiolabelling of glycosylated MFE-23::CPG2 fusion protein (MFECP1) with Tc-99m for quantitation of tumour antibody-enzyme localisation in antibody-directed enzyme pro-drug therapy (ADEPT). European J. Nuclear Med. Mol. Imaging 31, 1090–1096 (2004).
Mayer, A. et al. A phase I study of single administration of antibody-directed enzyme prodrug therapy (ADEPT) with the recombinant anti-CEA antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin. Cancer Res. (in press).
Bhatia, J. et al. Catalytic activity of an in vivo tumor targeted anti-CEA scFv::carboxypeptidase G2 fusion protein. Int. J. Cancer 85, 571–577 (2000).
Bretthauer, R.K. & Castellino, F.J. Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Biochem. 30, 193–200 (1999).
Trimble, R.B., Atkinson, P.H., Tschopp, J.F., Townsend, R.R. & Maley, F. Structure of oligosaccharides on Saccharomyces SUC2 invertase secreted by the methylotrophic yeast Pichia pastoris. J. Biol. Chem. 266, 22807–22817 (1991).
Medzihradszky, K.F. et al. Glycoforms obtained by expression in Pichia pastoris improve cancer targeting potential of a recombinant antibody-enzyme fusion protein. Glycobiology 14, 27–37 (2004).
Gerngross, T.U. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat. Biotechnol. 22, 1409–1414 (2004).
Malkin, E.M. et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect. Immun. 73, 3677–3685 (2005).
Herbst, R.S. et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J. Clin. Oncol. 20, 3792–3803 (2002).
Kobayashi, K. Summary of recombinant human serum albumin development. Biologicals 34, 55–59 (2006).
Li, H. et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24, 210–215 (2006).
Jahic, M., Gustavsson, M., Jansen, A.K., Martinelle, M. & Enfors, S.O. Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch processes. J. Biotechnol. 102, 45–53 (2003).
Sinha, J., Plantz, B.A., Inan, M. & Meagher, M.M. Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: case study with recombinant ovine interferon-τ. Biotechnol. Bioeng. 89, 102–112 (2005).
Kobayashi, K. et al. High-level expression of recombinant human serum albumin from the methylotrophic yeast Pichia pastoris with minimal protease production and activation. J. Biosci. Bioeng. 89, 55–61 (2000).
Wang, J. et al. Improved yield of recombinant merozoite surface protein 3 (MSP3) from Pichia pastoris using chemically defined media. Biotechnol. Bioeng. 90, 838–847 (2005).
Werten, M.W. et al. High-yield secretion of recombinant gelatins by Pichia pastoris. Yeast 15, 1087–1096 (1999).
Li, Z.J. et al. Low-temperature increases the yield of biologically active herring antifreeze protein in Pichia pastoris. Protein Expr. Purif. 21, 438–445 (2001).
Vervecken, W. et al. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 70, 2639–2646 (2004).
O'Leary, J.M. et al. Identification and removal of O-linked and non-covalently linked sugars from recombinant protein produced using Pichia pastoris. Protein Expr. Purif. 38, 217–227 (2004).
Acknowledgements
The authors would like to thank J. Bhatia, N. Woods and T. Hillyer for contributions to this manuscript. This work is supported by Cancer Research UK, The Royal Free Cancer Research Trust, The Copley May Foundation and The National Translational Cancer Research Network (NTRAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Note 1
Fermentor process log sheet (PDF 294 kb)
Supplementary Note 2
Aseptic handlings, Pichia spillage and disposal of waste (PDF 101 kb)
Supplementary Note 3
Calibration and maintenance of pH and DO probes (PDF 154 kb)
Supplementary Note 4
Guidelines for Pichia pastoris fermentation process development (PDF 127 kb)
Rights and permissions
About this article
Cite this article
Tolner, B., Smith, L., Begent, R. et al. Production of recombinant protein in Pichia pastoris by fermentation. Nat Protoc 1, 1006–1021 (2006). https://doi.org/10.1038/nprot.2006.126
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.126
This article is cited by
-
A thermostable and inhibitor resistant β-glucosidase from Rasamsonia emersonii for efficient hydrolysis of lignocellulosics biomass
Bioprocess and Biosystems Engineering (2024)
-
Predicting high recombinant protein producer strains of Pichia pastoris MutS using the oxygen transfer rate as an indicator of metabolic burden
Scientific Reports (2022)
-
Yeast-based bioproduction of disulfide-rich peptides and their cyclization via asparaginyl endopeptidases
Nature Protocols (2021)
-
Presence of protein production enhancers results in significantly higher methanol-induced protein production in Pichia pastoris
Microbial Cell Factories (2018)
-
Dynamic genome-scale metabolic modeling of the yeast Pichia pastoris
BMC Systems Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.