Abstract
We have used the high-affinity dopamine D-2 receptor radioligand, 18F-fallypride for evaluating receptor occupancy by the antipsychotic drugs, clozapine, risperidone, and haloperidol in rodents and nonhuman primates. In rodents, clozapine (0.1 mg/kg to 100 mg/kg) competed with 18F-fallypride at all the doses administered. At doses over 40 mg/kg, clozapine was able to displace all the administered 18F-fallypride. A pseudobiphasic profile of receptor occupancy by clozapine was observed. This behavior was compared with such other neuroleptics as risperidone and haloperidol that exhibited over 90% receptor occupancy at doses over 0.1 mg/kg and did not exhibit a biphasic nature. Dopamine D-2 receptor occupancy in the monkeys was studied using positron emission tomography (PET) after acute subcutaneous doses of the various drugs. At therapeutically relevant doses, clozapine, risperidone, and haloperidol were able to compete significantly with the binding of 18F-fallypride in all brain regions in rhesus monkeys, and our analyses indicate that these drugs (clozapine, risperidone, and haloperidol) do not discriminate between the striatal (caudate and putamen) and the extrastriatal (thalamus and cortical regions) dopamine receptors. The following extent of D-2 receptor occupancies were measured in the monkey brain using PET: clozapine ≈70% (dose of 9.7 mg/kg), risperidone ≈75% (0.05 mg/kg), and haloperidol ≈90% (0.05 mg/kg).
Similar content being viewed by others
Main
Clozapine, an atypical neuroleptic, has attracted particular attention because of its unique therapeutic properties (Schwartz and Brotman 1992). A number of studies comparing the efficacy of clozapine with other drugs have indicated the usefulness of this atypical drug in both positive and negative symptoms of schizophrenia (Safferman et al. 1991; Remington and Kapur 2000). The absence of extrapyramidal side effects (EPS) has been a significant factor in its efficacy (Rosenheck et al. 1997). This unique profile of clozapine has resulted in an ongoing effort to develop drugs that have the “clozapine pharmacological profile” (Schaus and Bymaster 1998).
Dopamine D-2 receptor blockade has been a primary mode of action for the typical and atypical neuroleptics (Sunahara et al. 1993; Seeman et al. 1997). A number of studies have investigated the extent of occupancy of the dopamine D-2 receptors in vivo in the striata (caudate and putamen) by various neuroleptics in patients undergoing treatment (summarized recently in Seeman and Tallerico 1999). These studies have been carried out using positron emission tomography (PET with 11C-raclopride and 11C-FLB 457 as the radioligand; for example, Farde et al. 1994, 1997; Kapur et al. 1999) as well as single photon emission tomography (SPECT using 123I-IBZM and 123I-epidepride; for example Kufferle et al. 1997; Pickar et al. 1996; Pilowsky et al. 1997). All studies report a high degree of dopamine D-2 receptor occupancy (60 to 90%) by various typical and atypical drugs (Seeman and Tallerico 1999). Two exceptions to the high degree of striatal occupancy are clozapine and quetiapine, which show occupancy levels generally not exceeding 60% (Seeman and Tallerico 1999; Kapur et al. 2000; Kufferle et al. 1997).
Clozapine has significant affinities for such other receptor systems as dopamine D-4 receptor, serotonergic system (particularly the 5HT-2a receptor), cholinergic and muscarinic systems, its effects on the glutamate system, which have all attracted much attention in an attempt to understand the therapeutic differences between clozapine and typical neuroleptics (Meltzer 1994). There is also the hypothesis that clozapine interacts differently at the nigrostriatal pathway versus the mesolimbic pathway and that this selective mesolimbic action accounts for the absence of the EPS effects (reviewed in Meltzer 1991). Results from in vivo imaging studies note that clozapine does not seem to increase occupancy of striatal D-2 receptors once it reaches a plateau of approximately 60%, unlike the >80% occupancy observed with the other high-affinity neuroleptics (e.g., in Kapur et al. 1999; Seeman and Tallerico 1999). In one report, a greater degree (>80%) of cortical D-2 receptor occupancy by clozapine has been reported using 123I-epidepride and SPECT (Pilowsky et al. 1997).
Our goal in this work was to address two issues. First, how does the in vivo occupancy of D-2 receptors vary with increasing doses of clozapine. Second, are there differential effects of clozapine in the striata (caudate and putamen) versus that in the extrastriata (thalamus, cortex, and other regions). For purposes of comparison, we also studied these two parameters for the newer atypical neuroleptic, risperidone, as well as the older typical neuroleptic haloperidol, both of which exhibit higher D-2 receptor occupancy as compared to clozapine (Kapur et al. 1999). We used rodents to study the dose-occupancy relationship and monkeys and PET to study striatal and extrastriatal occupancy. The high-affinity radioligand, 18F-fallypride, which has been shown to localize selectively at the D-2 receptors, both in the striata and extrastriata of rats and rhesus monkeys, was used to make these measurements (Mukherjee et al. 1999; Christian et al. 2000).
MATERIALS AND METHODS
Clozapine, risperidone, and haloperidol were purchased from Research Biochemicals Int. (Natick, MA). Production of 18F-fallypride, ((S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3′-18F-fluoropropyl)-2,3-dimethoxybenzamide) was carried out in the computer-controlled processor unit of the CTI RDS-112 cyclotron using modifications of our previously reported methods in specific activities of 2000 Ci/mmol (Mukherjee et al. 1999). Fluorine-18 radioactivity was counted in a Capintec dose calibrator, and low-level counting was carried out in a well counter (Auto-Gamma 5000, Packard Instruments Co.). Autoradiograms were read and analyzed using the Cyclone Storage Phosphor System (Packard Instruments Co.). Monkey PET studies were carried out using a high-resolution Siemens-ECAT HR+ scanner. All animal studies were approved by the Laboratory for Animal Care and Use Committee of the Wright State University.
Autoradiographic Studies
Male Sprague–Dawley rats (150–250 g) were anesthetized with diethyl ether and sacrificed; the brains were removed and frozen in isopentane previously cooled to −20°C. Coronal tissue sections (10 μm) of the brain were cut on a Lieca Cryocut 1850 cryostat and mounted on microscope slides previously cleaned in chromic acid and coated with gelatin. The tissue sections were then stored at −20°C until required.
Brain tissue sections were removed from storage and allowed to come to room temperature (22–25°C) over a period of 15 to 30 min. The tissue sections were placed in 50 mM Tris HCl buffer, pH 7.4, containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM NaEDTA, and 0.1 mM sodium ascorbate, and were preincubated for 15 min at 25°C. In the case of experiments with 5′-guanylylimidophosphate (Gpp(NH)p), which is known to convert the high-affinity (HA) sites to low-affinity (LA) sites (Grigoriadis and Seeman 1985), brain slices were preincubated for 15 min at 25°C with the above-mentioned buffer containing 50 μM of (Gpp(NH)p). The slices were then incubated with 18F-fallypride at concentrations of approximately 0.1 nM for 60 min at 25°C. Nonspecific binding was defined as the binding remaining in the presence of 10 μM (S)-sulpiride. Following incubation, tissue sections were washed twice for 0.5-min period each with cold 50 mM Tris HCl buffer, pH 7.4, followed by a quick rinse in cold deionized water. After washing, tissue sections were dried under a cool stream of air. Tissue sections were then apposed to storage phosphor screens (Packard Instruments Co.), and exposed for 2 h, and analyzed using the Cyclone Storage Phosphor System.
In Vivo Rodent Studies
Male Sprague–Dawley rats (200–250 g) were administered intraperitoneally with clozapine (0.1 mg/kg to 100 mg/kg), risperidone (0.001 mg/kg to 10 mg/kg), haloperidol (0.001 mg/kg to 10 mg/kg), or saline (for controls) 15 min before the administration of 18F-fallypride. Rats were then administered 18F-fallypride (200 μCi in 0.1 ml) by intracardiac injection under anesthesia (by exposure to vapors of diethyl ether). The rats were then allowed to recover from anesthesia and were allowed free access to food and water. Three hours postinjection of 18F-fallypride, the rats were killed, and the brains were excised and dissected. Striata and cerebellum were counted for fluorine-18 radioactivity to evaluate percentage-injected dose per gram. To ensure appropriate delivery of 18F-fallypride to the brain, in one group of rats, 18F-fallypride was administered 20 min before the administration of various doses of clozapine and killed at 3 h post-18F-fallypride injection.
In one set of experiments with different doses of clozapine, the striatal tissue concentration of clozapine was evaluated using reported methods (Baldessarini et al. 1993). Briefly, experiments were carried out as described above (i.e., groups of rats were administered 0, 10, 20, 40, 60, and 80 mg/kg of clozapine followed by 18F-fallypride and sacrificed 3 h postinjection of 18F-fallypride) and after counting for the amount of 18F-fallypride, the tissues were frozen for analysis. To measure extraction efficiency, 1 and 10 μg of clozapine were added to the control striata. All samples were homogenized with 1 cc of 0.5 M citric acid in a Potter–Elvejne homogenizer. This homogenate was then centrifuged at 10,000 rpm for 30 min in a Beckman Microfuge. The supernatant (0.6 cc) was mixed with 0.2 cc of 5 N sodium hydroxide and extracted with ethyl acetate (3 × 0.5 cc). The ethyl acetate was blown dry with nitrogen, and the sample was taken up in 0.1 cc of HPLC eluting buffer (68% acetonitrile and 0.25% triethylamine in water). Analysis of clozapine using a UV detector (at 254 nm) was carried out on a reverse-phase 5 μm Microsorb-MV C-18 column (250 × 4.6 mm) at a flow rate of 1 ml/min (retention time of clozapine was 6.8 min).
Occupancy of the D-2 receptor by the drug in each set of experiments was calculated as follows: ((St-Ce)C-(St-Ce)D)/(St-Ce)Cx100, where St is 18F-fallypride binding in striata, Ce is 18F-fallypride binding in cerebellum, (St-Ce)C represents specific binding of 18F-fallypride in the control striata, and (St-Ce)D represents specific binding of 18F-fallypride in the striata of rats with the drug challenge.
Monkey PET Studies
The male rhesus monkeys (8–10 kg) were anesthetized using ketamine (10 mg/kg) and were subsequently maintained on 0.5 to 1.5% isoflurane. The head of the monkey was placed in the gantry of a Siemens ECAT HR+ scanner and positioned in place with adhesive tape. After initial positioning, the animal was not moved for the duration of the scan. A transmission scan using a Ge-68/Ga-68 rod source was acquired before administration of the radiopharmaceutical. Image slices of the whole brain (in-plane spatial resolution of 4.5 mm at full width half maximum (FWHM)) parallel to the canthomeatal plane were acquired in three-dimensional (3-D) mode. Typically, a dynamic sequence of scans for a total of approximately 180 min were acquired immediately after intravenous administration of approximately 2 to 2.5 mCi of 18F-fallypride (methods described in detail in Christian et al. 2000). Arterial blood samples were drawn to provide input function for the studies. Several control studies were carried out by administering 18F-fallypride and graphic methods of quantitation of 18F-fallypride were applied as previously reported (Christian et al. 2000). To evaluate the effect of the various drugs in the monkeys, clozapine 9.7 mg/kg (29.7 μmoles), risperidone 0.05 mg/kg (0.12 μmoles), and haloperidol 0.05 mg/kg (0.13 μmoles) were administered subcutaneously, 18 to 20 min before the injection of 18F-fallypride. The monkeys were imaged for a period of 3 h subsequent to 18F-fallypride injection, and the dynamic data were analyzed as described below.
PET Data Analysis
Receptor occupancy by the drug in each set of experiments was calculated by measuring the change in a specific binding index. This index was measured by using the distribution volume method (DV) of 18F-fallypride in each brain region (Logan et al. 1990) as well as the distribution volume ratio method (DVR) using the cerebellum as a reference region (Logan et al. 1996) and are described below.
Method A: Distribution Volume (DV) (Logan et al. 1990)
This is a multiple time graphic method of analysis that yields a parameter representing the total (free, nonspecific, and specifically bound 18F-fallypride) distribution volume (DV) of the radioligand in a given region and is equal to K1/k2(1+NS+B′max/KD), where K1 and k2 are the plasma-to-tissue and tissue-to-plasma rate constants, B′max is the concentration of available receptor sites, NS is the ratio of binding constants for nonspecific binding of the radiotracer, and KD is the equilibrium dissociation rate constant (Logan et al. 1990; Volkow et al. 1999). Using a region such as the cerebellum and applying the method as outlined by Volkow et al. 1999, the percentage occupancy is calculated as (DVcont/DVcer−1)-(DVdrug/DVcer −1)/(DVcont/DVcer−1) × 100 where DVcont and DVdrug are the distribution volumes in a region-of-interest (caudate, putamen, thalamus, frontal cortex, or temporal cortex) in the control and drug challenge study, and DVcer is a predetermined estimate of the free plus nonspecific distribution volume, K1/k2(1+NS) calculated from compartmental modeling of the cerebellum in the control studies; a value of 0.97 for DVcer was used.
Method B: Distribution Volume Ratio (DVR) (Logan et al. 1996)
As with the DV method, this is also a multiple time graphic method that yields a parameter, DVR, that is proportional to specific binding. The DVR method differs from the DV method in that the reference region is incorporated rather than the arterial blood radioligand concentration (Logan et al. 1996). The implementation of this method to 18F-fallypride was previously described. This method assumes the existence of a reference region, such as the cerebellum, which is devoid of specific binding and has been used previously to measure transporter occupancies (Fowler et al. 1998). The drug occupancy using the DVR is calculated as: percentage occupancy = (1− [DVRdrug−1/(DVRcont−1]) × 100, where DVRcont is the distribution volume ratio of 18F-fallypride in the various brain regions of the control study, and DVRdrug is the distribution volume ratio of 18F-fallypride in the various brain regions of the drug study. The two methods described above were implemented to study the effect of possible small amounts of specific binding in the cerebellum. Although both methods use the cerebellum as a source for the reference distribution volume; for method A, the terms representing possible specific binding in the cerebellum have been removed by using compartment modeling to obtain K1/k2(1+NS).
RESULTS
In Vivo Rodent Studies
Various doses of clozapine were administered intraperitoneally to the rats (doses range from 0.1 mg/kg to 100 mg/kg). The rats on the lower doses did well; however, the rats at doses ≥60 mg/kg exhibited signs of catalepsy and some rats showed signs of convulsions at higher doses (≥80 mg/kg). More than 90% of the rats survived the high doses (≥60 mg/kg) of clozapine. In the rats 18F-fallypride was taken up rapidly in the brain and was consistent with our previously reported results (uptake in the striata of control rats in several experiments ranged between 1.5–2.0% injected dose/g of tissue; Mukherjee et al. 1999). As can be seen in Figure 1a, clozapine increasingly occupied the D-2 receptor sites in the striata. However, the rise in occupancy seemed to show two different plateaus. The first plateau was between doses 0.1 to 40 mg/kg and a subsequent sharp rise to the second plateau at doses between 40 to 100 mg/kg, which indicates that clozapine is able to almost completely occupy the D-2 receptors. This increase in occupancy of the D-2 receptors is attributable to the increase in brain concentrations of clozapine, as seen in the Figure 1b. This dose-dependent increase in brain clozapine levels has been previously reported (Baldessarini et al. 1993).
Plots showing extent of dopamine D-2 receptor occupancy measured using 18F-fallypride in the striata of male Sprague–Dawley rats treated with various doses of drugs: (a) clozapine; (b) striatal concentration of clozapine; (c) risperidone; and (d) haloperidol. Rats were injected intraperitoneally with various doses of the drugs 15 min before the intracardiac administration of 18F-fallypride. Three hours post-18F-fallypride administration, rats were sacrificed, and D-2 receptor occupancy was determined in the straita.
In all clozapine experiments, doses of clozapine were administered first followed by the administration of 18F-fallypride. However, in one set of clozapine experiments, to ascertain appropriate delivery of 18F-fallypride to the brain at higher doses, the series of experiments were carried out by first administering 18F-fallypride followed by the subsequent administration of clozapine. Results of this experiment were similar to the one described earlier, where clozapine was able to displace all the bound 18F-fallypride in the striata at higher doses.
The findings of clozapine are distinctly different from such drugs as risperidone and haloperidol, as shown in Figure 1c and d. At very low doses, both risperidone and haloperidol rapidly occupied the D-2 receptors and exhibited a steep rise, which is contrary to what was observed with clozapine. At doses of 0.1 mg/kg, risperidone and haloperidol exhibited occupancy in excess of 80%. Increases in doses beyond 0.1 mg/kg showed only a marginal change in occupancy in the case of risperidone; whereas, haloperidol showed almost a complete blockade of the receptors at higher doses. These findings are consistent with the higher affinity of risperidone (0.30 nM) and haloperidol (0.35 nM) for the D-2 receptors as compared to that of clozapine (44 nM) (Seeman et al. 1997).
In Vitro Autoradiographic Studies
Coronal sections of the rat brain exhibited a high degree of selective D-2 receptor binding of 18F-fallypride (Figure 2). Although 18F-fallypride has a significant affinity for the D-3 receptor subtype as well, no attempt was made in this study to decipher changes in the D-3 receptor binding of 18F-fallypride. Clozapine inhibited the binding of 18F-fallypride substantially at 100 nM (greater than 80% of 18F-fallypride was displaced), and at 10 nM clozapine, approximately 35 to 40% inhibition was observed; whereas, little inhibition was observed at 1 nM. To evaluate any potential effects on shifting the high-affinity state of the D-2 receptor to the low-affinity state competition experiments were carried out with clozapine in the presence of Gpp(NH)p. Total binding of 18F-fallypride was found to be insensitive to the effects of Gpp(NH)p. Extent of inhibition of 18F-fallypride by clozapine at various concentrations in the presence of Gpp(NH)p was found to be very similar to that measured in the absence of Gpp(NH)p.
Monkey PET Studies
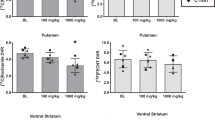
The PET studies in monkeys exhibited localization of 18F-fallypride in striatal and extrastriatal regions, as shown in Figure 3. The top row includes coronal slices showing caudate and putamen during the same time points (summed images between 115–175 min) for the different studies. As is evident from the images, significant reduction in the binding of 18F-fallypride occurs under drug challenge conditions. The lower row is a transaxial slice showing thalamus as well as cortical binding during the same time period, which is displaced upon pretreatment with neuroleptics. Figure 4 shows the time activity curves of the various regions (caudate, putamen, thalamus, frontal cortex, temporal cortex, and cerebellum) in the control monkey brain. Quantitation of the binding of 18F-fallypride in caudate, putamen, thalamus, frontal cortex, temporal cortex, and cerebellum in terms of distribution volumes and distribution volume ratios and are shown in Tables 1 and 2 (using previously described methods, Christian et al. 2000; Volkow et al. 1999). In the control monkey, putamen exhibited the highest degree of 18F-fallypride binding (DV = 48.1; DVR = 33.2) followed by the caudate (DV = 45; DVR = 30.8). The DV for thalamus was approximately 10% of that found in the caudate putamen and the cortex, temporal, and frontal, were about 5% of that found in the caudate. These findings are consistent with the dopamine receptor concentrations located extrastriatally with respect to that found in the striata (Hall et al. 1994; Kessler et al. 1993).
Monkey PET image slices (summed between 115 and 175 min) showing 18F-fallypride binding under control and drug challenge (clozapine, risperidone, and haloperidol) conditions. The top row shows brain coronal slices passing through caudate-putamen in control, clozapine, risperidone, and haloperidol studies. The bottom row shows transaxial slice passing through thalamus in the control, clozapine, risperidone, and haloperidol studies.
Occupancy Calculated Using the DV Method
The time-activity curves for the clozapine, risperidone, and haloperidol experiments are shown in Figures 5, 6, 7. Clozapine, at a therapeutically equivalent dose (9.7 mg/kg, administered subcutaneously, equivalent to approximately 700 mg/day), risperidone (0.05 mg/kg, subcutaneously, which is a therapeutically equivalent dose of approximately 3.5 mg/day), and haloperidol (0.05 mg/kg, subcutaneously, which is a therapeutically equivalent dose of approximately 3.5 mg/day) exhibited substantial degree of occupancy as measured by 18F-fallypride binding. Distribution volumes were evaluated by using the arterial input function to avoid the possible confounds of specific binding seen in the reference region (cerebellum). Occupancy evaluated using the distribution volumes are listed in Table 1. Clozapine exhibited 74% occupancy in caudate and 72% occupancy in putamen, and the extrastriatal regions were somewhat lower, exhibiting 65% for thalamus, 58% for the frontal cortex, 65% for the temporal cortex, and 79% for the cerebellum. For risperidone, occupancies were slightly higher: caudate and putamen exhibited 77 and 75%, respectively; whereas, thalamus exhibited 66%, and frontal cortex, temporal cortex, and cerebellum exhibited 56, 51, and 42%, respectively. Haloperidol exhibited highest occupancies, caudate and putamen showed 91 and 90%, respectively, and thalamus, cortex, and cerebellum ranged between 80% to being totally occupied.
Occupancy Calculated Using the DVR Method
The percentage dopamine receptor occupancy was evaluated from the DVR of control monkeys (DVRcont) and DVR of drug treated monkeys (DVRdrug) using the equation, (1−[DVRdrug−1/(DVRcont−1])× 100 and are shown in Table 2. In the case of clozapine, receptor occupancy in the striatal regions, caudate, and putamen were the highest, 63 and 65%, respectively. The extrastriatal regions were significantly lower, exhibiting 54% for thalamus and approximately 40% for the cortex. In the case of risperidone, caudate and putamen exhibited 73 and 70%; whereas, thalamus, frontal cortex, and temporal cortex exhibited 66, 61, and 42%, respectively. In the case of haloperidol, caudate and putamen showed 86 and 85%, respectively, and thalamus and cortex showed 88 and approximately 75% occupancy. In all cases, occupancy in the extrastriatal regions was found to be lower than the striatal regions.
DISCUSSION
Clozapine is an atypical neuroleptic with a moderate affinity for the D-2 receptors (Ki of 44 nM, Seeman et al. 1997; range of 60–152 nM, Wilson et al. 1998). Unlike the typical neuroleptics, which have a higher affinity for the D-2 receptors (<10 nM) therapeutic doses clozapine have been known to displace only up to approximately 50–60% of such bound radioligands as 11C-raclopride and 123I-IBZM (Seeman and Tallerico 1999). Our results, both in the rats and monkey, are in general agreement with the previous occupancy studies with clozapine at therapeutically relevant doses (i.e., up to approximately 10 mg/kg). At higher doses (i.e., >40 mg/kg), our results indicate that clozapine is able to occupy the D-2 receptors almost completely (>90%). At dose levels of 60 and 80 mg/kg, Figure 1b shows the concentration of clozapine in the striata to be significantly higher and is consistent with previous reports (Baldessarini et al. 1993). In the monkey PET study, we observed an occupancy of 72–74% (63 to 65%, using the DVR method) in the caudate and putamen. This is similar to what has been observed with 11C-raclopride and 123I-IBZM, but somewhat higher. It must be noted that our studies were carried out immediately after an acute dose; whereas, occupancies reported in patients are generally carried out at steady state after a few hours of the last dose. Quetiapine, a close analog of clozapine, was shown to exhibit significantly higher occupancies (58–64%) when the PET study was carried out 2 to 3 h after a single dose that declined to levels of 0 to 27% in 12 h (Kapur et al. 2000). A dose-dependent increase in occupancy of up to 87 to 89% with an IV dose of 20 mg/kg clozapine has also recently been reported in monkeys (Chou et al. 2000). Thus, it is evident that clozapine can occupy all D-2 receptors in the striatum in a dose-dependent manner in vivo. In human PET studies, however, because of limitations of amount of clozapine that may be administered, total occupancy in excess of 70% has not been possible (Kapur et al. 1999). Our PET findings on clozapine (72–74% occupancy for a dose of approximately 700 mg for a 70 kg subject) are in close proximity with the suggested 70–80% occupancy for a clozapine dose of 800 to 1,300 mg/day (Nyberg and Farde 2000).
In the case of risperidone, in vivo rat studies indicate a high degree of occupancy at fairly low doses. We observed a 50% occupancy of striatal D-2 receptors at a dose of <0.1 mg/kg. At a dose of 0.1 mg/kg risperidone was able to occupy in excess of 80% of the D-2 receptors. A previous study on the degree of occupancy of D-2 receptors in rats by risperidone has been reported using 125I-iodosulpiride (Leysen et al. 1993). This study found a significantly lower occupancy by risperidone as compared to our findings and reported a 50% occupancy at risperidone doses of 1.0 mg/kg administered subcutaneously in rodents. In PET studies with risperidone at a dose of 0.05 mg/kg, receptor occupancy calculated from DV and DVR values (Tables 1 and 2) show a 70 to 77% occupancy in the caudate and putamen. This observation of high striatal receptor occupancy is in agreement with the recent data on risperidone, which occupies greater than 70–80% of the D-2 receptors (Seeman and Tallerico 1999; Kapur et al. 1999; Kufferle et al. 1997). Our results also give further support to the recommendation of a risperidone dose of 2.5 to 3 mg/day to maintain adequate therapeutic D-2 occupancy (Nyberg and Farde 2000; Remington and Kapur 2000).
Such typical neuroleptics as haloperidol have been shown to occupy in excess of 80 to 90% of the D-2 receptors in human studies. In rat studies, low doses of haloperidol (0.05–0.1 mg/kg) were able to occupy a significant proportion of the D-2 receptors and were similar to that of risperidone. The PET study using a low dose (0.05 mg/kg) exhibited a 85 to 90% occupancy in the caudate and putamen. The high affinity of both haloperidol and risperidone for the D-2 receptors pose a significant challenge to maintain occupancy in the narrow window of 70 to 75%. These findings are consistent with the recommendations of clinical doses of haloperidol in the range of 2 to 3 mg/day to maintain occupancy below the 75 to 80% threshold level for EPS (Nyberg and Farde 2000; Remington and Kapur 2000).
Occupancy of Extrastriatal D-2 Receptors
The ability of 18F-fallypride to provide good extrastriatal localization in such areas as the thalamus, cortex, and other brain regions results from its high affinity and selectivity for the D-2 receptors (Mukherjee et al. 1999; Christian et al. 2000). Imaging methods for the related high-affinity substituted benzamides, 123I-epidepride and 11C-FLB 457 have also been developed to study extrastriatal binding at the D-2 receptors (Fujita et al. 1999; Delforge et al. 1999). It must be noted that these radiotracers also have a significant affinity for the less abundant D-3 receptor subtypes. In this report, we have not made any attempts to delineate D-3 receptor binding of 18F-fallypride. Figure 3 shows the selective localization of 18F-fallypride in the thalamus, and the time-activity curves in Figure 4 show the uptake and clearance of 18F-fallypride from the thalamus and cortical areas. This extrastriatal localization of 18F-fallypride provides us with an ability to evaluate the extent of D-2 receptor occupancy by clozapine, risperidone, and haloperidol.
The use of a reference region is frequently employed in the measurement of D-2 receptor occupancy in brain regions using PET and SPECT. For equilibrium studies, this region represents the free plus nonspecific radioligand concentration when measuring the bound to free ratios (Farde et al. 1997). For kinetic studies, a reference region is used to calculate an index of specific binding, such as binding potential (BP) or distribution volume ratio (DVR) (Fowler et al. 1998; Volkow et al. 1999). For this work, we investigated the use of the cerebellum as a reference region on the calculation of the receptor occupancy.
In employing reference region methods, such as the DVR, the measured occupancy in the high receptor regions of the striata were in close agreement with the DV measured values reported in Tables 1 and 2. However, in the extrastriatal regions of low receptor density, both the DV and DVR measured occupancies were found to be lower than striatal regions. A small, but significant, specific cerebellar binding in rats and our current studies have suggested drug-induced displacement of specific binding in the monkey cerebellum (Mukherjee et al. 1999). This has also been reported for such other high-affinity D-2 antagonists as 11C-FLB 457 (Delforge et al. 1999) and 125I-epidepride (Hall et al. 1996). Therefore, using the cerebellum as a reference tissue might have to be studied carefully to evaluate the extent of occupancy in regions of low receptor concentrations. The DVR approach would be suitable if a region within the cerebellum can be identified that contains a minimal amount of dopamine receptors and, thus, minimize specific 18F-fallypride binding in the reference region.
To avoid the confounds of small specific binding in the cerebellum, the receptor occupancy for this work was calculated by the use of distribution volumes (DV; Logan et al. 1990; Volkow et al. 1999) as well as the DVR method (Logan et al. 1996). Occupancy values obtained from the DV method (which minimizes the specific binding in the cerebellum) were found to be generally higher for all regions for the three drugs. As seen in Tables 1 and 2, using both the methods, occupancy values in the extrastriatal regions were similar or lower than the striatal regions for the three drugs. Therefore, this would suggest that in nonhuman primates at a fairly high dose, clozapine interacts with D-2 receptors in all brain regions to a similar extent. Using 11C-FLB 457, a similar striatal and thalamic occupancy level was reported by Farde et al. (1997).
Using the ratio analysis similar to that reported by Pilowsky et al. (1997), occupancy in the extrastriatal regions was found to be higher than striatal regions in the case of all the drugs, particularly for clozapine and risperidone. This finding is similar to the higher temporal cortex D-2 occupancy (90%) compared to that in the striata (58%) reported in patients on clozapine and measured using a similar ratio method with 123I-epidepride and SPECT (Pilowsky et al. 1997). Measurement of receptor occupancy by the ratio method is confounded by at least two factors: (1) because of the different kinetic profile of 18F-fallypride in the high versus low receptor concentration regions, the time at which pseudoequilibrium is achieved is different; and (2) the radiotracer likely reaches pseudoequilibrium in the drug challenge experiments at different times as compared to the control experiment and, therefore, measurement of occupancy using single time points may be difficult.
Can Endogenous Dopamine Affect D-2 Occupancy by Clozapine?
A significant finding in our studies relates to the difference between clozapine to that of risperidone and haloperidol in terms of occupying D-2 receptors. Risperidone and haloperidol being high-affinity dopamine D-2 receptor antagonists (Ki = 0.3 nM and 0.35 nM, respectively, Seeman et al. 1997), exhibited a rather steep increase in occupancy with escalating doses. However, clozapine, which has a moderate affinity for D-2 receptors (Ki = 44 nM, Seeman et al. 1997), exhibited an increase in occupancy but did not exhibit a steep rise and almost exhibited a tendency to plateau at approximately 40 to 60% occupancy before it went up to 90% occupancy with increasing doses. Clozapine has been shown to exhibit high brain concentrations and is significantly greater than in the plasma. Also, extraction fraction of clozapine from plasma seems to increase marginally at higher clozapine concentrations (Baldessarini et al. 1993). Our results indicate the increase in occupancy by clozapine is attributable to the increase in concentration of clozapine in the brain with increasing doses and that, at higher doses, there is a significantly greater concentration of the drug (Figure 1b).
It has been previously suggested that relatively high concentrations of dopamine in the striata may compete with such moderate affinity compounds as clozapine more so than with such higher-affinity compounds as risperidone (Seeman and Tallerico 1999; Wilson et al. 1998). Dopamine D-2 receptors exist in two interconvertible conformational states, depending on their G-protein associations, and these states are differentiated by the affinity with which they bind dopamine and are, thus, referred to as the high-affinity (HA) or the low-affinity state (LA) (Grigoriadis and Seeman 1985). For the HA-state, dopamine has an affinity of approximately 10 nM; whereas, for the LA-state, dopamine has an affinity of 5 μM (Seeman et al. 1985). Thus, competition of the various drugs with dopamine for the high-affinity state can be a significant issue. It has been suggested that 20% of the D-2 receptors are occupied by dopamine and 30% of the receptor sites are vulnerable to increases in dopamine (Laruelle 2000).
We have previously shown that binding of 18F-fallypride is not significantly altered in reserpinized rats compared to control rats, suggesting that basal levels of dopamine has little affect on 18F-fallypride binding to D-2 receptors (Mukherjee et al. 1997). However, when the concentration of dopamine is increased by pharmacological challenges of d-amphetamine, displacement of 18F-fallypride is observed. Clozapine on the other hand, having a significantly lower affinity and higher dissociation rate for the D-2 receptors (as compared to risperidone, haloperidol, and fallypride) is more susceptible to competition by basal dopamine levels. Thus, although both 18F-fallypride and clozapine bind to both, HA- and LA-state of the receptors, the difference in their dissociation rates allows dopamine to compete with clozapine at the HA-state more efficiently (Seeman and Tallerico 1999). This competition of dopamine with clozapine at the HA-state of the receptors may result in the transient occupancy nature of clozapine, as suggested by Kapur and Seeman (2000).
Because of this dopamine competition, clozapine may preferentially bind at the LA-state and then progressively occupy the HA-state as the concentration of clozapine is increased and at higher doses of clozapine, all D-2 receptors are occupied. Thus, it is possible that dopamine, because of its differential affinities for the HA- and LA-state, imposes a pseudobiphasic characteristic on clozapine. Thus, in PET studies with moderate doses of clozapine an occupancy higher than 60 to 70% is seldom seen. Thus, clozapine may differ from such higher affinity drugs as risperidone and haloperidol, in terms of sparingly occupying the high-affinity site as compared to the low-affinity sites of D-2 receptors.
D-2 Occupancy and Extrapyramidal Side Effects
It is generally believed that when neuroleptics exceed approximately 80% occupancy of the D-2 receptors, propensity of extrapyramidal side effects (EPS) increase. Clozapine has been shown not to cause EPS. Previous work from several laboratories has found clozapine to occupy approximately 60% of the D-2 receptors. It has been suggested that clozapine may exhibit “loose binding,” which may be attributable to the competition with the radioligand being used as well as with dopamine, and this may account for the low D-2 receptor occupancy seen with clozapine (Seeman et al. 1997; Seeman and Tallerico 1999; Wilson et al. 1998). Our findings in PET studies are in agreement with these occupancy levels. Based on our hypothesis of clozapine; that is, “high LA state and low HA state occupancy,” is it possible that EPS arises primarily from high occupancy of the HA state of the D-2 receptors? A more direct evidence of the extent of HA state occupancy will be worthwhile to further prove or disprove this hypothesis.
Limbic Selectivity
One unique feature of clozapine is its limbic selectivity; that is, a more selective inhibition of limbic versus striatal dopamine activity. Two major prevailing hypotheses include: (1) a differential inhibition of striatal and limbic dopamine D-2 receptors; and (2) a correctly balanced inhibition of several neurotransmitter receptors for obtaining limbic selectivity (reviewed in Arnt and Skarsfeldt 1998).
Our work has studied the first hypothesis of differential occupancy of D-2 receptors in the striatal and limbic areas. In this limited nonhuman primate occupancy study with clozapine, a similar extent of occupancy in striatal as well as the extrastriatal areas has been observed. Our analyses of the striatal areas (caudate and putamen) included, in part, the ventral striatum, which has been suggested to include the limbic regions in the nonhuman primate brain (Heimer et al. 1997; Haber and McFarland 1999). Our results of D-2 receptor occupancy in the various brain regions would suggest that the hypothesis of differential occupancy of D-2 receptors may not account for the limbic selectivity of clozapine in nonhuman primates. Although the dopamine D-2 receptor system has been shown to exhibit a high degree of homology between nonhuman primates and humans, PET studies using 18F-fallypride in human subjects undergoing treatment with clozapine will be useful in shedding further light on the issue of differential occupancy in D-2 receptors between striatal and limbic areas to account for the limbic selectivity.
CONCLUSIONS
Among the many questions about the unique aspects of clozapine's behavior as an atypical neuroleptic drug, we believe our results suggest the following. First, clozapine can compete with the binding of 18F-fallypride to dopamine D-2 receptors in the striata in rodents and can occupy all D-2 receptors in vivo. Second, at therapeutically relevant doses, clozapine can significantly compete with the binding of 18F-fallypride in all brain regions in the rhesus monkey. Preliminary analysis indicates a similar receptor occupancy by clozapine in the striata (caudate and putamen) and the extrastriata (thalamus and cortical regions) in the monkey brain. Third, it is postulated that high-affinity drugs, such as haloperidol and risperidone, bind to similar extent at the various conformational states of the receptor. However, moderate affinity drugs, such as clozapine may have a reduced occupancy at the high-affinity conformation of the receptor because of competition with endogenous dopamine. Because the high-affinity conformation is the functional state of the receptor, excessive occupancy of this conformation may lead to EPS effects.
References
Arnt J, Skarsfeldt T . (1998): Do novel antipsychotics have similar pharmacological characteristics? A review of evidence. Neuropsychopharmacology 18: 6–101
Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons D, Cohen BM . (1993): Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology 9: 117–124
Chou Y, Nyberg S, Halldin C . (2000): High occupancy of D-2 dopamine receptor by high doses of clozapine in the primate brain. J Nucl Med 41: 205
Christian BT, Shi B, Narayanan TK, Mukherjee J . (2000): Quantitation of striatal and extrastriatal dopamine D-2 receptors using PET imaging of F-18 fallypride in non-human primates. Synapse 38: 71–79
Delforge J, Bottlaender M, Loc'h C, Guenther I, Fuseau C, Bendriem B, Syrota A, Maziere B . (1999): Quantitation of extrastriatal D2 receptors using a very high-affinity ligand (FLB 457) and the multi-injection approach. J Cereb Blood Flow Metab 19: 533–546
Farde L, Nordstrom A-L, Nyberg S, Halldin S, Sedvall G . (1994): D-1, D-2, and 5HT-2 receptor occupancy in clozapine-treated patients. J Clin Psychiat 55: 67–69
Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C . (1997): A PET study of 11C-FLB 457 binding to extrastriatal D-2 dopamine receptors in healthy subjects and antipsychotic drug-related patients. Psychopharmacology 133: 396–404
Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding Y-S, Wang G-J . (1998): Measuring dopamine transporter occupancy by cocaine in vivo: Radiotracer considerations. Synapse 28: 111–116
Fujita M, Seibyl JP, Verhoeff NPLG, Ichise M, Baldwin RM, Zoghbi SS, Burger C, Staley JK, Rajeevan N, Charney DS, Innis RB . (1999): Kinetic and equilibrium analyses of 123I-epidepride binding to striatal and extrastriatal dopamine D2 receptors. Synapse 34: 290–304
Grigoriadis D, Seeman P . (1985): Complete conversion of brain D-2 dopamine receptors from the high- to low-affinity state for dopamine agonists, using sodium ions and guanine nucleotide. J Neurochem 44: 1925–1935
Haber SN, McFarland NR . (1999): The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci 877: 33–48
Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G . (1996): Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using 125I-epidepride. Synapse 23: 115–123
Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L . (1994): Distribution of D-1 and D-2 dopamine receptors and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11: 245–256
Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS . (1997): The accumbens: Beyond the core-shell dichotomy. J Neuropsych Clin Neurosci 9: 354–381
Kapur S, Seeman P . (2000): Transient occupancy at the D2 receptor—A new hypothesis for atypical antipsychotics. Biol Psychiat 47: 69S
Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P . (2000): A positron emission tomography study of quetiapine in schizophrenia. Arch Gen Psychiat 57: 553–559
Kapur S, Zipursky RB, Remington G . (1999): Clinical and theoretical implications of 5HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiat 156: 286–293
Kessler RM, Ansari S, Votaw JR, de Paulis T, Clanton JA, Schmidt DE, Mason NS, Manning RG . (1993): Identification of extrastriatal dopamine D-2 receptors in post-mortem human brain with 125I-epidepride. Brain Res 609: 237–243
Kufferle B, Tauscher J, Asenbaum S, Vesely C, Podreka I, Bruke T, Kasper S . (1997): IBZM SPECT imaging of striatal dopamine-2 receptors in psychotic patients treated with the novel antipsychotic substance quetiapine in comparison to clozapine and haloperidol. Psychopharmacology (Berl) 133: 323–328
Laruelle M . (2000): Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 20: 423–451
Leysen JE, Janssen PMF, Schotte A, Luyten WHML, Megens AAHP . (1993): Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: Role of 5HT2 receptors. Psychopharmacology 112: S40–S54
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hizemann R, Bendriem B, Gatley SJ, Christman DR . (1990): Graphical analysis of reversible radioligand binding from time-activity measurement applied to [N-11C-methyl]-(-)cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10: 740–747
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL . (1996): Distribution volume ratios without blood sampling from graphical analysis and PET data. J Cereb Blood Flow Metab 16: 834–840
Meltzer HY . (1991): The mechanism of action of novel antipsychotic drugs. Schizophr Bull 17: 263–287
Meltzer HY . (1994): An overview of the mechanism of action of clozapine. J Clin Psychiat 55: 47–52
Mukherjee J, Yang Z-Y, Lew R, Brown T, Kronmal S, Cooper M, Seiden LS . (1997): Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in non-human primates using positron emission tomography. Synapse 27: 1–13
Mukherjee J, Yang Z-Y, Brown T, Lew R, Wernick M, Ouyang X, Yasillo N, Chen C-T, Mintzer R, Cooper M . (1999): Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol 26: 519–527
Nyberg S, Farde L . (2000): Nonequipotent doses partly explain differences among antipsychotics—Implications of PET studies. Psychopharmacology 148: 22–23
Pickar D, Su TP, Weinberger DR, Coppola R, Malhotra AK, Knable MB, Lee KS, Gorey J, Bartko MB, Breier A, Hsiao J . (1996): Individual variation in D-2 dopamine receptor occupancy in clozapine-treated patients. Am J Psychiat 153: 1571–1578
Pilowsky LS, Mulligan RS, Acton PD, Ell PJ, Costa DC, Kerwin RW . (1997): Limbic selectivity of clozapine. Lancet 350: 490–491
Remington G, Kapur S . (2000): Atypical antipsychotics: Are some more atypical than others? Psychopharmacology 148: 3–15
Rosenheck R, Cramer J, Xu W, Thomas J, Henderson W, Frisman L, Fye C, Charney D . (1997): A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med 337: 809–815
Safferman A, Lieberman JA, Kane JM, Szymanski S, Kinon B . (1991): Update on the clinical efficacy and side effects of clozapine. Schizophr Bull 17: 247–261
Schaus JM, Bymaster FP . (1998): Dopaminergic approaches to antipsychotic agents. In Robertson DW (ed), Annual Reports in Medicinal Chemistry, San Diego: Academic Press, pp 1–10
Schwartz JT, Brotman AW . (1992): A clinical guide to antipsychotics. Drugs 44: 981–992
Seeman P, Corbett R, Van Tol HM . (1997): Atypical neuroleptics have low affinity for dopamine D-2 receptors or are selective for D-4 receptors. Neuropscyhopharmacology 16: 93–110
Seeman P, Watanabe M, Grigoriadis D, Tedesco JL, George SR, Svensson U, Nilsson JLG, Neumeyer JL . (1985): Dopamine D-2 receptor binding sites for agonists: A tetrahedral model. Mol Pharmacol 28: 391–399
Seeman P, Tallerico T . (1999): Rapid release of antipsychotic drugs from dopamine D-2 receptors: An explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiat 156: 876–884
Sunahara RK, Seeman P, Van Tol HHM, Niznik HB . (1993): Dopamine receptors and antipsychotic drug response. Br J Psychiat 163: 31–38
Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR . (1999): Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharm Exp Therapeut 291: 409–415
Wilson JM, Sanyal S, Van Tol HHM . (1998): Dopamine D-2 and D-4 receptor ligands: Relation to antipsychotic action. Euro J Pharmacol 351: 273–286
Acknowledgements
Financial support was provided by the U.S. Department of Energy DE-FG02-98ER62540. We thank Dr. Harold Stills for helpful discussions and Kelly Dunigan and Marilyn Brackney for technical assistance. We also acknowledge the Wallace-Kettering Neuroscience Institute and the Air Force Research Laboratory under Cooperative Agreement No. F33615-98-2-6002 for use of the PET scanner. Presented in part at the 29th Annual Meeting of the Society for Neuroscience, Miami, Florida, October 23–28, 1999 and Neuroreceptor Mapping Meeting, New York, June 9–11, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, J., Christian, B., Narayanan, T. et al. Evaluation of Dopamine D-2 Receptor Occupancy by Clozapine, Risperidone, and Haloperidol In Vivo in the Rodent and Nonhuman Primate Brain Using 18F-Fallypride. Neuropsychopharmacol 25, 476–488 (2001). https://doi.org/10.1016/S0893-133X(01)00251-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00251-2
Keywords
This article is cited by
-
Differential Effects of Neonatal Ventral Hippocampus Lesion on Behavior and Corticolimbic Plasticity in Wistar–Kyoto and Spontaneously Hypertensive Rats
Neurochemical Research (2023)
-
Behavioral and qEEG effects of the PDE10A inhibitor THPP-1 in a novel rhesus model of antipsychotic activity
Psychopharmacology (2016)
-
Clozapine, atypical antipsychotics, and the benefits of fast-off D2 dopamine receptor antagonism
Naunyn-Schmiedeberg's Archives of Pharmacology (2012)
-
Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex
Psychopharmacology (2011)
-
Differential Effects of Antipsychotic and Glutamatergic Agents on the phMRI Response to Phencyclidine
Neuropsychopharmacology (2008)