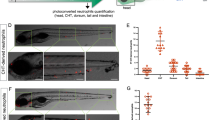
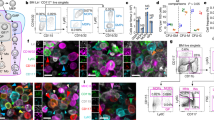
Abstract
Neutrophil granulocyte biology is a central issue of immunological research, but the lack of animal models that allow for neutrophil-selective genetic manipulation has delayed progress. By modulating the neutrophil-specific locus Ly6G with a knock-in allele expressing Cre recombinase and the fluorescent protein tdTomato, we generated a mouse model termed Catchup that exhibits strong neutrophil specificity. Transgene activity was found only in very few eosinophils and basophils and was undetectable in bone marrow precursors, including granulomonocytic progenitors (GMPs). Cre-mediated reporter-gene activation allowed for intravital two-photon microscopy of neutrophils without adoptive transfer. Homozygous animals were Ly6G deficient but showed normal leukocyte cellularity in all measured organs. Ly6G-deficient neutrophils were functionally normal in vitro and in multiple models of sterile or infectious inflammation in vivo. However, Cre-mediated deletion of FcγRIV in neutrophils reduced the cells' recruitment to immune-complex-mediated peritonitis, suggesting a cell-intrinsic role for activating Fc receptors in neutrophil trafficking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gunzer, M. Traps and hyper inflammation—new ways that neutrophils promote or hinder survival. Br. J. Haematol. 164, 189–199 (2014).
Köhler, A. et al. G-CSF mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117, 4349–4357 (2011).
Sundd, P. et al. 'Slings' enable neutrophil rolling at high shear. Nature 488, 399–403 (2012).
De Filippo, K. et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121, 4930–4937 (2013).
Lämmermann, T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013).
Nimmerjahn, F., Bruhns, P., Horiuchi, K. & Ravetch, J.V. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23, 41–51 (2005).
Kasperkiewicz, M. et al. Genetic identification and functional validation of FcgammaRIV as key molecule in autoantibody-induced tissue injury. J. Pathol. 228, 8–19 (2012).
Jakus, Z., Nemeth, T., Verbeek, J.S. & Mocsai, A. Critical but overlapping role of FcgammaRIII and FcgammaRIV in activation of murine neutrophils by immobilized immune complexes. J. Immunol. 180, 618–629 (2008).
Wojtasiak, M. et al. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 91, 2158–2166 (2010).
Stegemann, S. et al. Increased susceptibility for superinfection with Streptococcus pneumoniae during influenza virus infection is not caused by TLR7-mediated lymphopenia. PLoS One 4, e4840 (2009).
Wang, J.X. et al. Ly6G ligation blocks recruitment of neutrophils via a beta2-integrin-dependent mechanism. Blood 120, 1489–1498 (2012).
Yipp, B.G. & Kubes, P. Antibodies against neutrophil LY6G do not inhibit leukocyte recruitment in mice in vivo. Blood 121, 241–242 (2013).
Clausen, B.E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Faust, N., Varas, F., Kelly, L.M., Heck, S. & Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726 (2000).
Passegué, E., Wagner, E.F. & Weissman, I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 119, 431–443 (2004).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Becher, B. et al. High-dimensional analysis of the murine myeloid cell system. Nat. Immunol. 15, 1181–1189 (2014).
Szymczak, A.L. et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594 (2004).
Behnsen, J. et al. Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans. PLoS Pathog. 3, e13 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Elliott, E.R. et al. Deletion of Syk in neutrophils prevents immune complex arthritis. J. Immunol. 187, 4319–4330 (2011).
Abram, C.L., Roberge, G.L., Hu, Y. & Lowell, C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 408, 89–100 (2014).
King, K.Y. & Goodell, M.A. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 11, 685–692 (2011).
Hasenberg, M. et al. Rapid immunomagnetic negative enrichment of neutrophil granulocytes from murine bone marrow for functional studies in vitro and in vivo. PLoS One 6, e17314 (2011).
Drevets, D.A. & Campbell, P.A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect. Immun. 59, 2645–2652 (1991).
Hasenberg, M., Stegemann-Koniszewski, S. & Gunzer, M. Cellular immune reactions in the lung. Immunol. Rev. 251, 189–214 (2013).
Christy, A.L., Walker, M.E., Hessner, M.J. & Brown, M.A. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J. Autoimmun. 42, 50–61 (2013).
El Malki, K. et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J. Invest. Dermatol. 133, 441–451 (2013).
Seeling, M. et al. Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl. Acad. Sci. USA 110, 10729–10734 (2013).
Sachs, U.J. et al. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J. Biol. Chem. 282, 23603–23612 (2007).
Ribechini, E., Leenen, P.J. & Lutz, M.B. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol. 39, 3538–3551 (2009).
Bruns, S. et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6, e1000873 (2010).
Neumann, J. et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 129, 259–277 (2015).
Kim, J.V., Kang, S.S., Dustin, M.L. & McGavern, D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Ahn, G.O. et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc. Natl. Acad. Sci. USA 111, 2698–2703 (2014).
Uhm, T.G., Kim, B.S. & Chung, I.Y. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 4, 68–79 (2012).
Yang, W. et al. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 499, 491–495 (2013).
Kleiman, A. et al. Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J. 26, 722–729 (2012).
Thompson, A.A. et al. Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood 123, 366–376 (2014).
Sprüssel, A. et al. Lysine-specific demethylase 1 restricts hematopoietic progenitor proliferation and is essential for terminal differentiation. Leukemia 26, 2039–2051 (2012).
Heib, V. et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood 110, 946–953 (2007).
Acknowledgements
We thank C. Kurts (University of Bonn, Germany) for helpful discussions and Imaging Center Essen (IMCES; http://imces.uk-essen.de) for help with imaging. This work was supported by the German Research Foundation (DFG; “Immunobone” to M.G. (GU769/4-1, GU769/4-2), A.W. and F.N.; SFB854 to M.G. and B.S.), the European Union (EU HEALTH-2013-INNOVATION-1, MATHIAS to M.G.) and the Mercator Research Center Ruhr (to M.G.).
Author information
Authors and Affiliations
Contributions
M.G. conceived of and supervised the study and wrote the manuscript with the help of J.R.G., D.R.E. and S.B. A.H., M.H., L.M., F. Neumann, L.B., M. Stecher, A. Kraus, D.R.E., A. Klingberg, P.S., Z.A., S.K., S.E., A.R., M. Seeling, A.W., J.R.G. and F. Nimmerjahn performed experiments. S.B. provided crucial ideas. M.G., A.H., M.H., B.S. and J.R.G. discussed and interpreted results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Genotyping of Catchup mice.
(a) Targeting strategy for the generation of Catchup mice. (b) Genotyping of Catchup mice by PCR. Primer identification and position are given in a.
Supplementary Figure 2 Absence of tdTomato expression in other leukocytes of Catchup mice.
(a) Ex vivo two-photon imaging allows for the visualization of brain structure by the SHG signal of the meninges (brown) and the blood vessel system (CD31, blue). GFP-positive microglia (green) are positioned below the meninges in CX3CR1-eGFP brain. Whereas bone marrow of CatchupIVM-red mice showed brightly Ly6G-positive neutrophils (data not shown; compare Fig. 3 and Supplementary Fig. 5a) the brain was negative for Tomato-fluorescent cells. (b) The dense collagen network on the liver surface (SHG, brown) covered the blood vessel system (CD31, blue) and was therefore removed (right-hand images). Whereas CX3CR1-eGFP mice showed brightly fluorescent cells (green) in close proximity to the liver surface (SHG, brown), in CatchupIVM-red mice no Ly6G-positive cells were seen, but autofluorescent structures (red and blue) could be detected in the corresponding position within the liver. Data in a and b are representative of two CatchupIVM-red and three CX3CR1-eGFP mice. (c,d) Flow cytometric analysis of single-cell suspensions from the spleen (c) and the liver (d). Pseudo-colored dot plots show the gating strategy that was used to identify neutrophils (upper row in c) or macrophages (lower row in c and d). Histograms represent tdTomato expression of Ly6G+Ly6C+ neutrophils (upper histogram in c) and of Ly6G– cells (lower histogram in c) from the spleen of CatchupIVM-red mice versus C57BL/6 control animals. Histograms in d show tdTomato expression of MHCII+F4/80+ Kupffer cells from the liver of Catchup versus C57BL/6 control animals. Data are representative of three mice per group. (e,f) Gating strategy and tdTomato expression in blood eosinophils in CatchupIVM-red mice (e) and blood Basophils of Catchup (middle panel) and CatchupIVM-red mice (right panel) (f). The numbers indicate the percentage of positive cells from the group of eosinophils or basophils. Among all tdTomato+ cells, the total rate of cells with eosinophil characteristics was 0.3%–0.8%, and for those with basophil characteristics it was 0.3%–1%. Data in e are representative of two measured CatchupIVM-red mice. Data in f are from one homozygous Catchup mouse or representative of two measured CatchupIVM-red mice.
Supplementary Figure 3 Gating strategy for measuring neutrophil depletion without using Ly6G as a marker.
The first step in quantifying the neutrophil subset upon antibody-mediated depletion was to gate on living cells, identified by forward scatter (FSC) and side scatter (SSC) properties (a). By displaying the FSC height values over the FSC area values, we went on to exclude cell doublets (b). Next we chose CD45+ cells to exclusively analyze leukocytes (c). In homozygous Catchup animals we could not use the Ly6G signal to quantify neutrophils directly. Our idea for overcoming this problem was to use CD11b as a surface marker for neutrophils. Unfortunately CD11b is not specific for neutrophils and is also expressed on macrophages. Therefore we needed a strategy to exclude macrophages from the analysis. After a bone marrow cell suspension had been stained with the anti-Ly6G, anti-Ly6C antibody RB6-8C5, macrophages displayed a medium staining level. In the next step we used this fact to set a not-gate on this population, to sort out macrophages in the subsequent quantification plot (d). Finally we assumed that all CD11b+ cells among the remaining cells were neutrophils, and we calculated their number as a percentage of the total number of cells. Back-gating on the FSC/SSC properties of this cell population demonstrated that these cells displayed the common neutrophil-like profile (e).
Supplementary Figure 4 Characterization of leukocytes from Catchup mice.
(a) TdTomato expression by the Catchup allele (Ly6g(Cre-tom)) and catchup-Cre-mediated tdTomato expression from a reporter allele (ROSA26(Rtom)) relative to surface expression levels of Ly6G and CD11b in bone marrow cells. Extended representation of data from Figure 2a. (b) Quantification of Cre-recombined cells (tdTomato+) within bone marrow populations of CatchupIVM-red mice expressing increasing levels of surface Ly6G. Populations defined according to gating strategy in a. Data are mean + s.e.m. from three analyzed animals. (c) Flow-cytometric analysis of peripheral blood leukocytes from CatchupIVM-red mice. Gating and overlay analysis demonstrating that tdTomato-expressing cells (magenta dots) showed a uniform FSC/SSC profile and the high Ly6G surface-expression characteristic of neutrophils. CD115+ monocytic cells (turquoise dots) showed a distinct FSC/SSC profile and were Ly6G–. Representative plots are shown. (d) Alternative gating strategy for the analysis in c: most peripheral Ly6G+ cells were tdTomatobright, whereas CD115+ cells were tdTomato– regardless of their Ly6C expression level.
Supplementary Figure 5 Bone marrow cells positive for tdTomato uniformly express surface Ly6G and CD11b.
Representative plots are shown (n = 3, CatchupIVM-red mice).
Supplementary Figure 6 Analysis of blood and spleen transgene activity according to surface expression of CD11b and Ly6G.
The same gating strategy as shown for bone marrow cells in Figure 2a was used to determine tdTomato expression in CD11b+Ly6G+ subpopulations of peripheral blood and spleen. Representative plots of different genotypes are shown.
Supplementary Figure 7 Cytospin morphology of sorted tdTomato+ cells.
Bone marrow cells of either Catchup or CatchupIVM-red mice were FACS-sorted according to the strategy shown in a. In the first step just cells of an FSC/SSC profile matching all living cells were included in the sorting process. Subsequently cell doublets were excluded by analysis of the FSC-area/FSC-height properties. Catchup neutrophils were then identified by their distinct tdTomato expression. Neutrophils from CatchupIVM-red mice were further subdivided into cells with low or high tdTomato expression. An equally measured sample of C57BL/6 animals shows that the tdTomato low and high gates were devoid of a neutrophil-characteristic dot distribution. (b) Catchup bone marrow cells were characterized before and after FACS. The upper left picture shows an overlay of a widefield image and the corresponding tdTomato-fluorescence channel; the picture in the lower left shows just the tdTomato channel. The micrograph in the upper right visualizes a representative Wright-stained cytospin sample of the particular cell suspension. In parallel, the cells were analyzed by flow cytometry regarding their CD45 and tdTomato expression (lower right). The red events in the FSC/SSC dot plot represent all CD45/tdTomato double-positive cells. (c) The same characterization was conducted for CatchupIVM-red bone marrow cells including the two neutrophil subpopulations exhibiting differently strong tdTomato levels. Scale bars, 50 µm. Data are representative of two Catchup and three CatchupIVM-red animals analyzed in two independent sorting runs. In one run, one wild-type animal was also analyzed as a control.
Supplementary Figure 8 Intracellular signaling in Ly6G– neutrophils is normal.
(a) Freshly isolated neutrophils from wild-type (WT) and Ly6G-deficient mice were either stimulated with PMA (100 ng/ml) for 30 min (dashed line) or left unstimulated (solid line). The thin line shows the isotype control. Erk phosphorylation was determined by intracellular FACS staining. One representative of three independent experiments is shown. (b) Phosphorylation of Erk was quantified by calculating the ratio of the MFI from stimulated/unstimulated neutrophils. Shown is the mean ± s.d. of three independent experiments. (c) Western blot analysis of neutrophils from wild-type and Ly6g–/– mice. 3 × 106 freshly isolated neutrophils from wild-type and Ly6g–/– mice were either stimulated with PMA (100 ng/ml) for 10 and 30 min or left unstimulated. The blots were reprobed with the indicated antibodies. (d) Quantification of the western blot in c. Shown is one representative experiment of three. Each experiment used pooled bone marrow neutrophils from two individual mice per group.
Supplementary Figure 9 Infiltration of neutrophils in the spinal cord during the onset of EAE and the lung during invasive aspergillosis.
(a) Flow cytometric analysis of neutrophil distribution in the spinal cords of wild-type (WT) and Ly6G-deficient mice on day 14 of EAE. Shown is the percentage (left panel) and the absolute cell number (right panel) of neutrophils in the CD45+CD11b+ population. Neutrophils of wild-type mice were identified by their Ly6G expression; those of Ly6G-deficient mice were detected by their expression of tdTomato. Non-significant differences were determined with Student’s t-test (wild type, n = 6 mice; Ly6G-deficient, n = 8 mice; P > 0.5). (b) Numbers of neutrophils (CD45+Ly6G+ or tdTomato+) recruited to the lung in uninfected mice or in mice 6 and 24 h after intratracheal A. fumigatus infection. The result is the mean + s.d. of four individual experiments after 6 h and 24 h of infection for six Ly6g–/– and six Ly6g+/+ mice per time point and of three individual experiments with three Ly6g–/– and three Ly6g+/+ uninfected animals. (c) CFU of A. fumigatus in the lungs of infected mice at different time points after infection. Data are mean ± s.d. for three animals per group and time point.
Supplementary Figure 10 Ly6g–/– mice fend off a systemic Candida infection.
Ly6G-competent (Ly6g+/+, black line) and Ly6G-deficient (Ly6g–/–, red dashed line) mice were intravenously injected with a sublethal dose of Candida albicans. At the time points indicated, the percent survival (upper graph) and the percent weight loss were determined. The number of investigated animals is given in the figure.
Supplementary Figure 11 Survival of Ly6G– neutrophils in vitro and in vivo is normal.
(a) Development of death in neutrophils in vitro. Bone marrow neutrophils were incubated in cell-culture dishes for different time intervals, stained with anti-Ly6G (clone 1A8, Horizon V450), annexin V (FITC) and propidium iodide (PI) and analyzed using flow cytometry. Shown is the percentage of PI-positive events in neutrophil suspensions from Ly6g–/–, Ly6g+/– and Ly6g+/+ mice after the indicated time points. The experiment was done once. (b–d) The bone marrow of Ly6G-competent (CD45.2+Ly6G+) and Ly6G-deficient (CD45.2+Ly6G–) mice were mixed in a 1:1 ratio and transplanted into lethally irradiated congenic CD45.1+ recipient mice (b). After the generation of these mixed bone marrow chimeras, the abundance of CD45.2+Ly6G– cells (revealed by CD45.2 antibody staining and tdTomato expression) and CD45.2+Ly6G+ cells (revealed by CD45.2 and Ly6G antibody staining) was analyzed by flow cytometry at the time points indicated (c,d). Six bone marrow–transplanted animals were analyzed.
Supplementary Figure 12 Efficiency of gene deletion by the Cre system.
(a,b) Analysis of the number (mean + s.d.) of circulating resident monocytes and neutrophils (a) in control mice (6 R4flox and 11 R4-deficient animals), LysM-CreR4flox mice (6 animals) and CatchupR4flox mice (6 animals) and their FcγRIV expression by flow cytometry (b) In both used Cre mice, a reduction in the expression of FcγRIV on neutrophils of about 50% could be detected (b, left). Whereas FcγRIV expression levels on resident monocytes were reduced by about 80% in LysM-CreR4flox mice, normal expression could be shown for CatchupR4flox mice (b, right). Data are from eight R4flox, nine CatchupR4flox, six LysM-CreR4flox and six R4-deficient animals. (c) Determination of FcγRIV expression of spleen neutrophils by FACS analysis. Data are mean + s.d. of 13 C57Bl/6, 12 CatchupR4flox and 8 R4-deficient mice. (d) FcγRIV gene expression analysis by qPCR from 50 ng RNA of neutrophils sorted from the spleen. Shown are means + s.d. of two (CatchupR4flox) or three sorted samples. (e) Quantitative real-time reverse transcriptase-PCR (RT-PCR) from tdTomato-positive cells of the indicated genotypes of mice was performed for Il1r2. Expression levels are shown relative to those of the housekeeping gene HPRT1 for IL-1R-2 and to Actb for FcγRIV.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Table 1 (PDF 1243 kb)
Distribution of neutrophils in the bone marrow.
Tibial bone marrow was subjected to intravital microscopy in a Catchup/Rosa26-CAG-tdTomato double transgenic animal (CatchupIVM-red). The movie shows a 3D rendering of a high-resolution Z-Stack (134 μm thick with 1-μm step size in Z) with the visualization of calcified bone (gray, second harmonic generation (SHG)), blood vessels (green) and neutrophils (red). (MOV 6753 kb)
Migration of neutrophils in the bone marrow of a resting mouse.
Tibial bone marrow was subjected to intravital microscopy in a CatchupIVM-red animal that was not stimulated before the experiment was performed. The movie shows a 30-min time series in which neutrophils (red) and blood vessels (green) were visualized simultaneously. Each frame is an extended focus of 24 layers in Z with 4-μm spacing between layers. The real time of the experiment is shown on the lower right. (MOV 2423 kb)
Migration of neutrophils in the bone marrow of a mobilized mouse.
Tibial bone marrow was subjected to intravital microscopy in a CatchupIVM-red animal that had received an injection of G-CSF 2 h earlier. The movie shows a 30-min time series in which neutrophils (red) and blood vessels (green) were visualized simultaneously. Each frame is an extended focus of 26 layers in Z with a 4-μm spacing between layers. The real time of the experiment is shown on the lower right. (MOV 2397 kb)
Long-term observation of neutrophil migration in situ.
In situ imaging of a thinned CatchupIVM-red tibia via two-photon microscopy shows the long time stability of the tomato fluorescence, with a slightly decreasing intensity of about 5% per hour. The low migratory activity of the cells is due to a lack of G-CSF stimulation in the animal, as well as to the decay of cell viability at the end of the long experiment. The SHG intensity increased as a result of sample shift. The fluorescence decay curve is given in the last frame of the video. The real time of the experiment is shown on the lower right. (MOV 20497 kb)
Migration of neutrophils in vitro.
Neutrophil granulocytes were isolated from the marrow of either wild-type or homozygous Catchup (i.e., Ly6Gk-deficient) mice. Their spontaneous and fMLP-induced migration was recorded by time-lapse video microscopy in a temperature-controlled chamber. The real time (hh:mm:ss.ttt) of the experiment is shown on the lower right. (MOV 5315 kb)
Rights and permissions
About this article
Cite this article
Hasenberg, A., Hasenberg, M., Männ, L. et al. Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat Methods 12, 445–452 (2015). https://doi.org/10.1038/nmeth.3322
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3322
This article is cited by
-
Experimental hepatic encephalopathy causes early but sustained glial transcriptional changes
Journal of Neuroinflammation (2023)
-
NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury
Nature Metabolism (2023)
-
A subset of type-II collagen-binding antibodies prevents experimental arthritis by inhibiting FCGR3 signaling in neutrophils
Nature Communications (2023)
-
A cardioimmunologist’s toolkit: genetic tools to dissect immune cells in cardiac disease
Nature Reviews Cardiology (2022)
-
The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury
Nature Communications (2022)