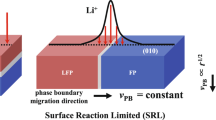
Abstract
Many battery electrodes contain ensembles of nanoparticles that phase-separate on (de)intercalation. In such electrodes, the fraction of actively intercalating particles directly impacts cycle life: a vanishing population concentrates the current in a small number of particles, leading to current hotspots. Reports of the active particle population in the phase-separating electrode lithium iron phosphate (LiFePO4; LFP) vary widely, ranging from near 0% (particle-by-particle) to 100% (concurrent intercalation). Using synchrotron-based X-ray microscopy, we probed the individual state-of-charge for over 3,000 LFP particles. We observed that the active population depends strongly on the cycling current, exhibiting particle-by-particle-like behaviour at low rates and increasingly concurrent behaviour at high rates, consistent with our phase-field porous electrode simulations. Contrary to intuition, the current density, or current per active internal surface area, is nearly invariant with the global electrode cycling rate. Rather, the electrode accommodates higher current by increasing the active particle population. This behaviour results from thermodynamic transformation barriers in LFP, and such a phenomenon probably extends to other phase-separating battery materials. We propose that modifying the transformation barrier and exchange current density can increase the active population and thus the current homogeneity. This could introduce new paradigms to enhance the cycle life of phase-separating battery electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J-M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Ohzuku, T., Iwakoshi, Y. & Sawai, K. Formation of lithium–graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 140, 2490–2498 (1993).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
Tang, M., Carter, W. C. & Chiang, Y-M. Electrochemically driven phase transitions in insertion electrodes for lithium-ion batteries: Examples in lithium metal phosphate olivines. Annu. Rev. Mater. Res. 40, 501–529 (2010).
Ohzuku, T., Ueda, A. & Yamamoto, N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 142, 1431–1435 (1995).
Ariyoshi, K., Iwakoshi, Y., Nakayama, N. & Ohzuku, T. Topotactic two-phase reactions of Li[Ni1/2Mn3/2]O4(P4332) in nonaqueous lithium cells. J. Electrochem. Soc. 151, A296–A303 (2004).
Woodford, W. H., Chiang, Y-M. & Carter, W. C. “Electrochemical shock” of intercalation electrodes: A fracture mechanics analysis. J. Electrochem. Soc. 157, A1052–A1059 (2010).
Christensen, J. & Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 10, 293–319 (2006).
Yamada, A. et al. Room-temperature miscibility gap in LixFePO4 . Nature Mater. 5, 357–360 (2006).
Meethong, N., Huang, H-Y. S., Carter, W. C. & Chiang, Y-M. Size-dependent lithium miscibility gap in nanoscale Li1−xFePO4 . Electrochem. Solid-State Lett. 10, A134–A138 (2007).
Wagemaker, M. et al. Dynamic solubility limits in nanosized olivine LiFePO4 . J. Am. Chem. Soc. 133, 10222–10228 (2011).
Malik, R., Abdellahi, A. & Ceder, G. A critical review of the Li insertion mechanisms in LiFePO4 electrodes. J. Electrochem. Soc. 160, A3179–A3197 (2013).
Delmas, C., Maccario, M., Croguennec, L., Le Cras, F. & Weill, F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nature Mater. 7, 665–671 (2008).
Brunetti, G. et al. Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction. Chem. Mater. 23, 4515–4524 (2011).
Sugar, J. D. et al. High-resolution chemical analysis on cycled LiFePO4 battery electrodes using energy-filtered transmission electron microscopy. J. Power Sources 246, 512–521 (2014).
Chueh, W. C. et al. Intercalation pathway in many-particle LiFePO4 electrode revealed by nanoscale state-of-charge mapping. Nano Lett. 13, 866–872 (2013).
Dreyer, W. et al. The thermodynamic origin of hysteresis in insertion batteries. Nature Mater. 9, 448–453 (2010).
Laffont, L. et al. Study of the LiFePO4/FePO4 two-phase system by high-resolution electron energy loss spectroscopy. Chem. Mater. 18, 5520–5529 (2006).
Badi, S-P. et al. Direct synthesis of nanocrystalline Li0.90FePO4: Observation of phase segregation of anti-site defects on delithiation. J. Mater. Chem. 21, 10085–10093 (2011).
Ferguson, T. R. & Bazant, M. Z. Phase transformation dynamics in porous battery electrodes. Electrochim. Acta (in the press)
Srinivasan, V. & Newman, J. Discharge model for the lithium iron–phosphate electrode. J. Electrochem. Soc. 151, A1517–A1529 (2004).
Yu, D. Y. W., Donoue, K., Inoue, T., Fujimoto, M. & Fujitani, S. Effect of electrode parameters on LiFePO4 cathodes. J. Electrochem. Soc. 153, A835–A839 (2006).
Dargaville, S. & Farrell, T. W. Predicting active material utilization in LiFePO4 electrodes using a multiscale mathematical model. J. Electrochem. Soc. 157, A830–A840 (2010).
Prada, E. et al. Simplified electrochemical and thermal model of LiFePO4-graphite Li-ion batteries for fast charge applications. J. Electrochem. Soc. 159, A1508–A1519 (2012).
Ferguson, T. R. & Bazant, M. Z. Nonequilibrium thermodynamics of porous electrodes. J. Electrochem. Soc. 159, A1967–A1985 (2012).
Bai, P. & Tian, G. Statistical kinetics of phase-transforming nanoparticles in LiFePO4 porous electrodes. Electrochim. Acta 89, 644–651 (2013).
Levi, M. D. et al. Collective phase transition dynamics in microarray composite LixFePO4 electrodes tracked by in situ electrochemical quartz crystal admittance. J. Phys. Chem. C 117, 15505–15514 (2013).
Bai, P. & Bazant, M. Z. Charge transfer kinetics at the solid–solid interface in porous electrodes. Nature Commun. 5, 517–557 (2014).
Orvananos, B. et al. Architecture dependence on the dynamics of nano-LiFePO4 electrodes. Electrochim. Acta 137, 245–257 (2014).
Zhang, X. et al. Rate-induced solubility and suppression of the first-order phase transition in olivine LiFePO4 . Nano Lett. 14, 2279–2285 (2014).
Liu, H. et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 344, 1252817 (2014).
Newman, J. & Thomas-Alyea, K. E. Electrochemical Systems 517–577 (Wiley, 2004).
Bazant, M. Z. Theory of chemical kinetics and charge transfer based on nonequilibrium thermodynamics. Acc. Chem. Res. 46, 1144–1160 (2013).
Bluhm, H. et al. Soft X-ray microscopy and spectroscopy at the molecular environmental science beamline at the advanced light source. J. Electron Spectrosc. Relat. Phenom. 150, 86–104 (2006).
Kilcoyne, A.L.D. et al. Interferometer-controlled scanning transmission X-ray microscopes at the advanced light source. J. Synchrotron Radiat. 10, 125–136 (2003).
Kilcoyne, D. et al. in 10th Int. Conf. Synchrotron Radiat. Instrum. (eds Garrett, R., Gentle, I., Nugent, K. & Wilkins, S.) 465–468 (American Institute of Physics, 2010).
Liu, X. et al. Phase transformation and lithiation effect on electronic structure of LixFePO4: An in-depth study by soft X-ray and simulations. J. Am. Chem. Soc. 134, 13708–13715 (2012).
Cogswell, D. A. & Bazant, M. Z. Theory of coherent nucleation in phase-separating nanoparticles. Nano Lett. 13, 3036–3041 (2013).
Gibot, P. et al. Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4 . Nature Mater. 7, 741–747 (2008).
Bai, P., Cogswell, D. A. & Bazant, M. Z. Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett. 11, 4890–4896 (2011).
Malik, R., Zhou, F. & Ceder, G. Kinetics of non-equilibrium lithium incorporation in LiFePO4 . Nature Mater. 10, 587–590 (2011).
Yu, X. et al. High rate delithiation behaviour of LiFePO4 studied by quick X-ray absorption spectroscopy. Chem. Commun. 48, 11537–11539 (2012).
Cahn, J. W. On spinodal decomposition. Acta Metall. 9, 795–801 (1961).
Allen, S. M. & Cahn, J. W. A microscopic theory for antiphase boundary motion and its application to antiphase domain coarsening. Acta Metall. 27, 1085–1095 (1979).
Cogswell, D. A. & Bazant, M. Z. Coherency strain and the kinetics of phase separation in LiFePO4 nanoparticles. ACS Nano 6, 2215–2225 (2012).
Morgan, D., Van der Ven, A. & Ceder, G. Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem. Solid-State Lett. 7, A30–A32 (2004).
Srinivasan, V. & Newman, J. Existence of path-dependence in the LiFePO4 electrode. Electrochem. Solid-State Lett. 9, A110–A114 (2006).
Orvananos, B., Ferguson, T. R., Yu, H-C., Bazant, M. Z. & Thornton, K. Particle-level modeling of the charge-discharge behavior of nanoparticulate phase-separating Li-ion battery electrodes. J. Electrochem. Soc. 161, A535–A546 (2014).
Gaberscek, M., Küzma, M. & Jamnik, J. Electrochemical kinetics of porous, carbon-decorated LiFePO4 cathodes: Separation of wiring effects from solid state diffusion. Phys. Chem. Chem. Phys. 9, 1815–1820 (2007).
Meethong, N., Huang, H-Y. S., Speakman, S. A., Carter, W. C. & Chiang, Y-M. Strain accommodation during phase transformations in olivine-based cathodes as a materials selection criterion for high-power rechargeable batteries. Adv. Funct. Mater. 17, 1115–1123 (2007).
Omenya, F. et al. Why substitution enhances the reactivity of LiFePO4 . Chem. Mater. 25, 85–89 (2013).
Ravnsbæk, D. B. et al. Extended solid solutions and coherent transformations in nanoscale olivine cathodes. Nano Lett. 14, 1484–1491 (2014).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Park, K. et al. Enhanced charge-transfer kinetics by anion surface modification of LiFePO4 . Chem. Mater. 24, 3212–3218 (2012).
Acknowledgements
The research at Stanford was supported by the Samsung Advanced Institute of Technology Global Research Outreach Program, and by startup funding from Stanford School of Engineering and Precourt Institute for Energy. Support for the research at MIT was provided by the Samsung-MIT Program for Materials Design in Energy Applications. F.E.G. and N.C.B. were supported by the Office of Basic Energy Sciences, Division of Materials and Engineering Sciences, US Department of Energy, under contract DE-AC04-94AL85000. J.D.S. and K.R.F. were supported by US Department of Energy through the Sandia Laboratory Directed Research and Development program under contract DE-AC04-94AL85000. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. Beam line 5.3.2.1 at the Advanced Light Source was funded through a donation by the King Abdullah University of Science and Technology. Y.L. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-114747. We acknowledge M. Homer of Sandia and J. Perrino of Stanford for ultramicrotoming. We thank J. Nelson Weker of the Stanford Synchrotron Radiation Lightsource for insightful discussions.
Author information
Authors and Affiliations
Contributions
W.C.C., F.E.G. and Y.L. conceived the experiments. K.R.F. and F.E.G. prepared the LFP samples for imaging. Y.L., T.T. and A.L.D.K. performed the SoC imaging. J.D.S. and Y.L. performed the TEM imaging. Y.L. analysed the active particle population from the images. T.R.F., R.B.S., D.A.C. and M.Z.B. conceived and created the phase-field porous electrode model. W.C.C. and M.Z.B. supervised the project. All authors participated in writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1957 kb)
Rights and permissions
About this article
Cite this article
Li, Y., El Gabaly, F., Ferguson, T. et al. Current-induced transition from particle-by-particle to concurrent intercalation in phase-separating battery electrodes. Nature Mater 13, 1149–1156 (2014). https://doi.org/10.1038/nmat4084
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4084
This article is cited by
-
Chemical-state distributions in charged LiCoO2 cathode particles visualized by soft X-ray spectromicroscopy
Scientific Reports (2023)
-
Thermodynamics of multi-sublattice battery active materials: from an extended regular solution theory to a phase-field model of LiMnyFe1-yPO4
npj Computational Materials (2023)
-
The role of solid solutions in iron phosphate-based electrodes for selective electrochemical lithium extraction
Nature Communications (2022)
-
Correlative image learning of chemo-mechanics in phase-transforming solids
Nature Materials (2022)
-
Anomalous interfacial stress generation and role of elasto-plasticity in mechanical failure of Si-based thin film anodes of Li-ion batteries
Bulletin of Materials Science (2022)