Abstract
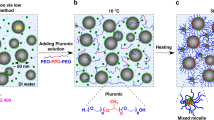
We report the formation of mesoporous organohydrogels from oil-in-water nanoemulsions containing an end-functionalized oligomeric gelator in the aqueous phase. The nanoemulsions exhibit an abrupt thermoreversible transition from a low-viscosity liquid to a fractal-like colloidal gel of droplets with mesoscale porosity and solid-like viscoelasticity with moduli approaching 100 kPa, possibly the highest reported for an emulsion-based system. We hypothesize that gelation is brought about by temperature-induced interdroplet bridging of the gelator, as shown by its dependence on the gelator chemistry. The use of photocrosslinkable gelators enables the freezing of the nanoemulsion’s microstructure into a soft hydrogel nanocomposite containing a large fraction of dispersed liquid hydrophobic compartments, and we show its use in the encapsulation and release of lipophilic biomolecules. The tunable structural, mechanical and optical properties of these organohydrogels make them a robust material platform suitable for a wide range of applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bell, C. L. & Peppas, N. A. Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-g-ethylene glycol). Biomaterials 17, 1203–1218 (1996).
Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 85, 1–33 (2000).
Eddington, D. T. & Beebe, D. J. Flow control with hydrogels. Adv. Drug Deliv. Rev. 56, 199–210 (2004).
Lee, K. Y. & Mooney, D. J. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1879 (2001).
Langer, R. & Tirrell, D. A. Designing materials for biology and medicine. Nature 428, 487–492 (2004).
Peppas, N. A., Hilt, J. Z., Khademhosseini, A. & Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360 (2006).
Vintiloiu, A. & Leroux, J. C. Organogels and their use in drug delivery—a review. J. Control. Release 125, 179–192 (2008).
Jung, J. H., Ono, Y. & Shinkai, S. Novel silica structures which are prepared by transcription of various superstructures formed in organogels. Langmuir 16, 1643–1649 (2000).
Ajayaghosh, A., Praveen, V. K. & Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 37, 109–122 (2008).
Panda, P. et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 8, 1056–1061 (2008).
Gombotz, W. R. & Wee, S. F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 31, 267–285 (1998).
Hoare, T. R. & Kohane, D. S. Hydrogels in drug delivery: Progress and challenges. Polymer 49, 1993–2007 (2008).
Lee, M. N. & Mohraz, A. Bicontinuous macroporous materials from Bijel templates. Adv. Mater. 22, 4836–4841 (2010).
Mason, T. G., Wilking, J. N., Meleson, K., Chang, C. B. & Graves, S. M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 18, R635–R666 (2006).
Wooster, T. J., Golding, M. & Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 24, 12758–12765 (2008).
Winter, H. H. & Chambon, F. Analysis of linear viscoelasticity of a cross-linking polymer at the gel point. J. Rheol. 30, 367–382 (1986).
Coussot, P. Rheophysics of pastes: A review of microscopic modelling approaches. Soft Matter. 3, 528–540 (2007).
Mason, T. G., Bibette, J. & Weitz, D. A. Yielding and flow of monodisperse emulsions. J. Colloid Interface Sci. 179, 439–448 (1996).
Wilking, J. N. & Mason, T. G. Irreversible shear-induced vitrification of droplets into elastic nanoemulsions by extreme rupturing. Phys. Rev. E 75, 041407 (2007).
Luisi, P. L., Scartazzini, R., Haering, G. & Schurtenberger, P. Organogels from water-in-oil microemulsions. Colloid Polym. Sci. 268, 356–374 (1990).
Filali, M. et al. Robust phase behavior of model transient networks. J. Phys. Chem. B 105, 10528–10535 (2001).
Wu, H., Xie, J. J., Lattuada, M. & Morbidelli, M. Scattering structure factor of colloidal gels characterized by static light scattering, small-angle light scattering, and small-angle neutron scattering measurements. Langmuir 21, 3291–3295 (2005).
Dibble, C. J., Kogan, M. & Solomon, M. J. Structure and dynamics of colloidal depletion gels: Coincidence of transitions and heterogeneity. Phys. Rev. E 74, 050401 (2006).
Bagger-Jörgensen, H., Coppola, L., Thuresson, K., Olsson, U. & Mortensen, K. Phase behavior, microstructure, and dynamics in a nonionic microemulsion on addition of hydrophobically end-capped poly(ethylene oxide). Langmuir 13, 4204–4218 (1997).
Michel, E., Filali, M., Aznar, R., Porte, G. & Appell, J. Percolation in a model transient network: Rheology and dynamic light scattering. Langmuir 16, 8702–8711 (2000).
Porte, G., Ligoure, C., Appell, J. & Aznar, R. Bridging interactions due to telechelic linkers balanced by screened Coulombic repulsions. J. Stat. Mech. Theory Exp. P05005 (2006).
Bhatia, S. R. & Russel, W. B. End-capped associative polymer chains between nanospheres: Attractions in ideal solutions. Macromolecules 33, 5713–5720 (2000).
Cabane, B. Structure of some polymer detergent aggregates in water. J. Phys. Chem. 81, 1639–1645 (1977).
Cabane, B. & Duplessix, R. Organization of surfactant micelles adsorbed on a polymer molecule in water—a neutron-scattering study. J. Physique 43, 1529–1542 (1982).
Li, Y. J., Dubin, P. L., Havel, H. A., Edwards, S. L. & Dautzenberg, H. Complex-formation between polyelectrolyte and oppositely charged mixed micelles—soluble complexes vs coacervation. Langmuir 11, 2486–2492 (1995).
Xia, J. L., Dubin, P. L. & Kim, Y. S. Complex-formation between poly(oxyethylene) and sodium dodecyl-sulfate micelles—light-scattering, electrophoresis, and dialysis equilibrium studies. J. Phys. Chem. 96, 6805–6811 (1992).
Helgeson, M. E. & Wagner, N. J. Colloidal interactions mediated by end-adsorbing polymer-like micelles. J. Chem. Phys. 135, 084901 (2011).
Rueb, C. J. & Zukoski, C. F. Viscoelastic properties of colloidal gels. J. Rheol. 41, 197–218 (1997).
Dawson, K. A. The glass paradigm for colloidal glasses, gels, and other arrested states driven by attractive interactions. Curr. Opin. Colloid Interface Sci. 7, 218–227 (2002).
Cates, M. E., Fuchs, M., Kroy, K., Poon, W. C. K. & Puertas, A. M. Theory and simulation of gelation, arrest and yielding in attracting colloids. J. Phys. Condens. Matter 16, S4861–S4875 (2004).
Zaccarelli, E. Colloidal gels: Equilibrium and non-equilibrium routes. J. Phys. Condens. Matter 19, 323101 (2007).
Kroy, K., Cates, M. E. & Poon, W. C. K. Cluster mode-coupling approach to weak gelation in attractive colloids. Phys. Rev. Lett. 92, 148302 (2004).
Chen, Y. L. & Schweizer, K. S. Microscopic theory of gelation and elasticity in polymer-particle suspensions. J. Chem. Phys. 120, 7212–7222 (2004).
Mason, T. G., Graves, S. M., Wilking, J. N. & Lin, M. Y. Effective structure factor of osmotically deformed nanoemulsions. J. Phys. Chem. B 110, 22097–22102 (2006).
Kawada, H. et al. Structure and rheology of a self-standing nanoemulsion. Langmuir 26, 2430–2437 (2009).
Chen, S. H., Rouch, J., Sciortino, F. & Tartaglia, P. Static and dynamic properties of water-in-oil microemulsions near the critical and percolation points. J. Phys. Condens. Matter 6, 10855–10883 (1994).
Sprakel, J., van der Gucht, J., Stuart, M. A. C. & Besseling, N. A. M. Brownian particles in transient polymer networks. Phys. Rev. E 77, 061502 (2008).
Helgeson, M. E. et al. Formation and rheology of viscoelastic double networks in wormlike micelle–nanoparticle mixtures. Langmuir 26, 8049–8060 (2010).
Helgeson, M. E., Chapin, S. C. & Doyle, P. S. Hydrogel microparticles from lithographic processes: Novel materials for fundamental and applied colloid science. Curr. Opin. Colloid Interface Sci. 16, 106–117 (2011).
Cates, M. E. & Clegg, P. S. Bijels: A new class of soft materials. Soft Matter 4, 2132–2138 (2008).
Herzig, E. M., White, K. A., Schofield, A. B., Poon, W. C. K. & Clegg, P. S. Bicontinuous emulsions stabilized solely by colloidal particles. Nature Mater. 6, 966–971 (2007).
Mitragotri, S. & Lahann, J. Physical approaches to biomaterial design. Nature Mater. 8, 15–23 (2009).
Kolishetti, N. et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl Acad. Sci. USA 107, 17939–17944 (2010).
Meleson, K., Graves, S. & Mason, T. G. Formation of concentrated nanoemulsions by extreme shear. Soft Matter 2, 109–123 (2004).
Kline, S. R. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 39, 895–900 (2006).
Acknowledgements
We acknowledge financial support from the Novartis-MIT Center for Continuous Manufacturing, as well as the support of the National Institute of Standards and Technology, US Department of Commerce, in providing the neutron research facilities used in this work, supported in part by the National Science Foundation under agreement DMR-0454672. P.S.D. and H.Z.A. acknowledge support from the Institute for Collaborative Biotechnologies through grant W911NF-09-0001 from the US Army Research Office. The content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. We thank G. McKinley for the use of rheometry equipment in this work.
Author information
Authors and Affiliations
Contributions
M.E.H. and P.S.D. designed the experiments. M.E.H. and S.E.M. prepared the nanoemulsions and carried out rheology, neutron scattering and photopolymerization experiments. M.E.H. carried out analysis of SANS/USANS data. M.E.H. and H.Z.A. carried out flow lithography and triggered release studies. M.E.H., S.E.M., H.Z.A. and P.S.D. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1269 kb)
Rights and permissions
About this article
Cite this article
Helgeson, M., Moran, S., An, H. et al. Mesoporous organohydrogels from thermogelling photocrosslinkable nanoemulsions. Nature Mater 11, 344–352 (2012). https://doi.org/10.1038/nmat3248
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3248
This article is cited by
-
Reconfigurable droplet networks
Nature Communications (2024)
-
Employing Nanoemulsions in Food Packaging: Shelf Life Enhancement
Food Engineering Reviews (2021)
-
Nanoemulsions for health, food, and cosmetics: a review
Environmental Chemistry Letters (2021)
-
Multiple nanoemulsions
Nature Reviews Materials (2020)
-
Thermoresponsive nanoemulsion-based gel synthesized through a low-energy process
Nature Communications (2019)