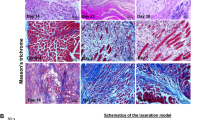
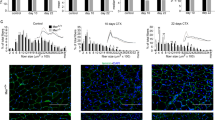
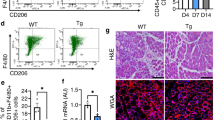
Abstract
Depending on the inflammatory milieu, injury can result either in a tissue's complete regeneration or in its degeneration and fibrosis, the latter of which could potentially lead to permanent organ failure. Yet how inflammatory cells regulate matrix-producing cells involved in the reparative process is unknown. Here we show that in acutely damaged skeletal muscle, sequential interactions between multipotent mesenchymal progenitors and infiltrating inflammatory cells determine the outcome of the reparative process. We found that infiltrating inflammatory macrophages, through their expression of tumor necrosis factor (TNF), directly induce apoptosis of fibro/adipogenic progenitors (FAPs). In states of chronic damage, however, such as those in mdx mice, macrophages express high levels of transforming growth factor β1 (TGF-β1), which prevents the apoptosis of FAPs and induces their differentiation into matrix-producing cells. Treatment with nilotinib, a kinase inhibitor with proposed anti-fibrotic activity, can block the effect of TGF-β1 and reduce muscle fibrosis in mdx mice. Our findings reveal an unexpected anti-fibrotic role of TNF and suggest that disruption of the precisely timed progression from a TNF-rich to a TGF-β−rich environment favors fibrotic degeneration of the muscle during chronic injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mann, C.J. et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 1, 21 (2011).
Joe, A.W.B. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 (2010).
Uezumi, A. et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143–152 (2010).
Lemos, D.R. et al. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells 30, 1152–1162 (2012).
Uezumi, A. et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 124, 3654–3664 (2011).
Chazaud, B. et al. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc. Sport Sci. Rev. 37, 18–22 (2009).
Brigitte, M. et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 62, 268–279 (2010).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Murray, P.J. & Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Lu, H. et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 (2011).
Pinto, A.R. et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS ONE 7, e36814 (2012).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 109, E3186–E3195 (2012).
Summan, M. et al. Macrophages and skeletal muscle regeneration: a clodronate–containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1488–R1495 (2006).
Ruffell, D. et al. A CREB–C/EBPβ cascade induces M2 macrophage–specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 106, 17475–17480 (2009).
Mounier, R. et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 18, 251–264 (2013).
Rhee, C.K. et al. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration 82, 273–287 (2011).
Liu, Y. et al. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J. Hepatol. 55, 612–625 (2011).
Taki, H. et al. Interstitial pneumonitis associated with infliximab therapy without methotrexate treatment. Rheumatol. Int. 30, 275–276 (2009).
Ostor, A.J., Crisp, A.J., Somerville, M.F. & Scott, D.G. Fatal exacerbation of rheumatoid arthritis associated fibrosing alveolitis in patients given infliximab. Br. Med. J. 329, 1266 (2004).
Huggett, M.T. & Armstrong, R. Adalimumab-associated pulmonary fibrosis. Rheumatology (Oxford) 45, 1312–1313 (2006).
Warren, G.L. et al. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 19, 413–415 (2005).
Duffield, J.S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Shireman, P.K. et al. MCP–1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 81, 775–785 (2007).
Tidball, J.G. & Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 (2010).
Kharraz, Y., Guerra, J., Mann, C.J., Serrano, A.L. & Muñoz–Cánoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013, 491497 (2013).
Mann, P.B., Elder, K.D., Kennett, M.J. & Harvill, E.T. Toll-like receptor 4–dependent early elicited tumor necrosis factor alpha expression is critical for innate host defense against Bordetella bronchiseptica. Infect. Immun. 72, 6650–6658 (2004).
Kruglov, A.A. & Nedospasov, S.A. Comment on “experimental arthritis triggers periodontal disease in mice: involvement of TNF-α and the oral microbiota.”. J. Immunol. 188, 4–5 (2012).
Kalajzic, I. et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J. Bone Miner. Res. 17, 15–25 (2002).
Wang, C.Y., Mayo, M.W., Korneluk, R.G., Goeddel, D.V. & Baldwin, A.S. Jr. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 (1998).
Bergmann, M.W., Loser, P., Dietz, R. & von Harsdorf, R. Effect of NF-κB Inhibition on TNF-α–induced apoptosis and downstream pathways in cardiomyocytes. J. Mol. Cell. Cardiol. 33, 1223–1232 (2001).
Bulfield, G., Siller, W.G., Wight, P.A. & Moore, K.J. X chromosome–linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 81, 1189–1192 (1984).
Porter, J.D. et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 11, 263–272 (2002).
Villalta, S.A., Nguyen, H.X., Deng, B., Gotoh, T. & Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496 (2009).
Desguerre, I. & Arnold, L. et al. A new model of experimental fibrosis in hindlimb skeletal muscle of adult mdx mouse mimicking muscular dystrophy. Muscle Nerve 45, 803–814 (2012).
Kramann, R., Dirocco, D.P. & Humphreys, B.D. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J. Pathol. 231, 273–289 (2013).
Liu, Y. et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 17, 1594–1601 (2011).
Peng, C.F. et al. Overexpression of cellular repressor of E1A-stimulated genes inhibits TNF-α–induced apoptosis via NF-κB in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 406, 601–607 (2011).
Distler, J.H.W., Schett, G., Gay, S. & Distler, O. The controversial role of tumor necrosis factor α in fibrotic diseases. Arthritis Rheum. 58, 2228–2235 (2008).
Chen, K., Wei, Y., Sharp, G.C. & Braley–Mullen, H. Decreasing TNF-α results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J. Leukoc. Biol. 81, 306–314 (2007).
Zhang, K., Gharaee-Kermani, M., McGarry, B., Remick, D. & Phan, S.H. TNF-α–mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J. Immunol. 158, 954–959 (1997).
Hodgetts, S., Radley, H., Davies, M. & Grounds, M.D. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFα function with Etanercept in mdx mice. Neuromuscul. Disord. 16, 591–602 (2006).
Grounds, M.D. & Torrisi, J. Anti-TNFα (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 18, 676–682 (2004).
Bermudez, L.E. & Young, L.S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-γ, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140, 3006–3013 (1988).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9, 271–285 (2009).
Vidal, B. et al. Fibrinogen drives dystrophic muscle fibrosis via a TGFβ /alternative macrophage activation pathway. Genes Dev. 22, 1747–1752 (2008).
Lawrance, I.C. et al. A murine model of chronic inflammation–induced intestinal fibrosis down-regulated by antisense NF-κB. Gastroenterology 125, 1750–1761 (2003).
Brack, A.S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Huang, P., Zhao, X.S., Fields, M., Ransohoff, R.M. & Zhou, L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 23, 2539–2548 (2009).
Dadgar, S.Z. et al. Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy. J. Cell Biol. 207, 139–158 (2014).
Richeldi, L. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 (2014).
Daniels, C.E. et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am. J. Respir. Crit. Care Med. 181, 604–610 (2010).
Heredia, J.E. et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013).
Grivennikov, S.I. et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005).
Acknowledgements
We thank the Biomedical Research Centre Animal Facility and core staff as well as the University of British Columbia flow cytometry facility staff for their technical assistance. We are very grateful to Claudia Hopkins for schematic presented in Supplementary Figure 10. The Col1a1*3.6-eGFP mice were a gift from D.W. Rowe (Center for Regenerative Medicine and Skeletal Development, University of Connecticut Health Center). This work was supported by a grant from the Heart and Stroke Foundation of Canada, the Canadian Institute for Health Research grant MOP 97856 (both to F.M.V.R.), and a Russian Science Foundation grant #14-50-00029 (to S.A.N.). F.B. was supported by a Four-Year Doctoral Fellowship (4YF) by the University of British Columbia, and M.L. was supported by a fellowship from the Chilean government.
Author information
Authors and Affiliations
Contributions
D.R.L. designed, directed and carried out experiments; analyzed data; and wrote the manuscript. F.B., M.L., C.-K.C., S.T.L., D.F., R.-H.Z. and A.N. carried out experiments and analyzed data. S.A.N. provided important advice on experimental design. F.M.V.R. designed experiments, directed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 & Supplementary Tables 1–3 (PDF 36097 kb)
Rights and permissions
About this article
Cite this article
Lemos, D., Babaeijandaghi, F., Low, M. et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21, 786–794 (2015). https://doi.org/10.1038/nm.3869
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3869
This article is cited by
-
MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury
Cellular and Molecular Life Sciences (2024)
-
Imaging mass cytometry analysis of Becker muscular dystrophy muscle samples reveals different stages of muscle degeneration
Scientific Reports (2024)
-
Stage-specific nutritional management and developmental programming to optimize meat production
Journal of Animal Science and Biotechnology (2023)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)
-
Myopathologic trajectory in Duchenne muscular dystrophy (DMD) reveals lack of regeneration due to senescence in satellite cells
Acta Neuropathologica Communications (2023)