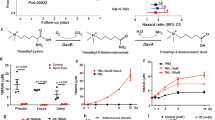
Abstract
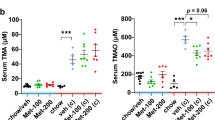
Intestinal microbiota metabolism of choline and phosphatidylcholine produces trimethylamine (TMA), which is further metabolized to a proatherogenic species, trimethylamine-N-oxide (TMAO). We demonstrate here that metabolism by intestinal microbiota of dietary l-carnitine, a trimethylamine abundant in red meat, also produces TMAO and accelerates atherosclerosis in mice. Omnivorous human subjects produced more TMAO than did vegans or vegetarians following ingestion of l-carnitine through a microbiota-dependent mechanism. The presence of specific bacterial taxa in human feces was associated with both plasma TMAO concentration and dietary status. Plasma l-carnitine levels in subjects undergoing cardiac evaluation (n = 2,595) predicted increased risks for both prevalent cardiovascular disease (CVD) and incident major adverse cardiac events (myocardial infarction, stroke or death), but only among subjects with concurrently high TMAO levels. Chronic dietary l-carnitine supplementation in mice altered cecal microbial composition, markedly enhanced synthesis of TMA and TMAO, and increased atherosclerosis, but this did not occur if intestinal microbiota was concurrently suppressed. In mice with an intact intestinal microbiota, dietary supplementation with TMAO or either carnitine or choline reduced in vivo reverse cholesterol transport. Intestinal microbiota may thus contribute to the well-established link between high levels of red meat consumption and CVD risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bernstein, A.M. et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation 122, 876–883 (2010).
Micha, R., Wallace, S.K. & Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 121, 2271–2283 (2010).
Siri-Tarino, P.W., Sun, Q., Hu, F.B. & Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 91, 535–546 (2010).
Bibbins-Domingo, K. et al. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 362, 590–599 (2010).
Hansen, E.S. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/2. Shared risk factors for cancer and atherosclerosis–a review of the epidemiological evidence. Mutat. Res. 239, 163–179 (1990).
Turnbaugh, P.J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Turnbaugh, P.J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Goodman, A.L. & Gordon, J.I. Our unindicted coconspirators: human metabolism from a microbial perspective. Cell Metab. 12, 111–116 (2010).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Bremer, J. Carnitine—metabolism and functions. Physiol. Rev. 63, 1420–1480 (1983).
Rebouche, C.J. & Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 18, 39–61 (1998).
Brass, E.P. Carnitine and sports medicine: Use or abuse? Ann. NY Acad. Sci. 1033, 67–78 (2004).
Stanley, C.A. Carnitine deficiency disorders in children. Ann. NY Acad. Sci. 1033, 42–51 (2004).
Demarquoy, J. et al. Radioisotopic determination of l-carnitine content in foods commonly eaten in western countries. Food Chem. 86, 137–142 (2004).
Rigault, C., Mazue, F., Bernard, A., Demarquoy, J. & Le Borgne, F. Changes in l-carnitine content of fish and meat during domestic cooking. Meat Sci. 78, 331–335 (2008).
Ley, R.E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008).
Muegge, B.D. et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974 (2011).
Zimmer, J. et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 66, 53–60 (2012).
Wu, G.D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Ley, R.E. et al. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102, 11070–11075 (2005).
Febbraio, M. et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056 (2000).
Suzuki, H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 (1997).
Brown, M.S. & Goldstein, J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Spann, N.J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Rader, D.J. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 92, 42J–49J (2003).
Jia, L., Betters, J.L. & Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 73, 239–259 (2011).
Schwarz, M., Russell, D.W., Dietschy, J.M. & Turley, S.D. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39, 1833–1843 (1998).
Repa, J.J. et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J. Biol. Chem. 275, 39685–39692 (2000).
Gulewitsch, W. & Krimberg, R. Zur Kenntnis der Extrakivstoffe der Muskein, II. Mitteilung. Uber das Carnitin. Hoppe-Seyler's Z. Physiol. Chem. 45, 326–330 (1905).
Rebouche, C.J., Mack, D.L. & Edmonson, P.F. l-Carnitine dissimilation in the gastrointestinal tract of the rat. Biochemistry 23, 6422–6426 (1984).
Rebouche, C.J. & Chenard, C.A. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J. Nutr. 121, 539–546 (1991).
Zhang, A.Q., Mitchell, S.C. & Smith, R.L. Dietary precursors of trimethylamine in man: a pilot study. Food Chem. Toxicol. 37, 515–520 (1999).
Delany, J.P., Snook, J.T., Vivian, V.M. & Cashmere, K. Metabolic effects of a carnitine-free diet fed to college students. Fed. Proc. 45, 815 (1986).
Zeisel, S.H., Mar, M.H., Howe, J.C. & Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 133, 1302–1307 (2003).
Fraser, G.E. Vegetarian diets: what do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 89, 1607S–1612S (2009).
Key, T.J. et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 70, 516S–524S (1999).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. published online, http://dx.doi.org/10.1056/NEJMoa1200303 (25 February 2013).
Brown, M.S. & Goldstein, J.L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science 185, 61–63 (1974).
Charach, G., Rabinovich, A., Argov, O., Weintraub, M. & Rabinovich, P. The role of bile Acid excretion in atherosclerotic coronary artery disease. Int. J. Vasc. Med. 2012, 949672 (2012).
Charach, G. et al. Decreased fecal bile acid output in patients with coronary atherosclerosis. J. Med. 29, 125–136 (1998).
Lu, Y., Feskens, E.J., Boer, J.M. & Muller, M. The potential influence of genetic variants in genes along bile acid and bile metabolic pathway on blood cholesterol levels in the population. Atherosclerosis 210, 14–27 (2010).
Miyake, J.H. et al. Transgenic expression of cholesterol-7-α-hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler. Thromb. Vasc. Biol. 22, 121–126 (2002).
Post, S.M., de Crom, R., van Haperen, R., van Tol, A. & Princen, H.M. Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arterioscler. Thromb. Vasc. Biol. 23, 892–897 (2003).
Zong, C. et al. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. (Maywood) 237, 194–200 (2012).
Altmann, S.W. et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Liberles, S.D. & Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 442, 645–650 (2006).
Suska, A., Ibanez, A.B., Lundstrom, I. & Berghard, A. G protein–coupled receptor mediated trimethylamine sensing. Biosens. Bioelectron. 25, 715–720 (2009).
Bai, C., Biwersi, J., Verkman, A.S. & Matthay, M.A. A mouse model to test the in vivo efficacy of chemical chaperones. J. Pharmacol. Toxicol. Methods 40, 39–45 (1998).
Mello, C.C. & Barrick, D. Measuring the stability of partly folded proteins using TMAO. Protein Sci. 12, 1522–1529 (2003).
Cordain, L. et al. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 81, 341–354 (2005).
Liszt, K. et al. Characterization of bacteria, clostridia and Bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann. Nutr. Metab. 54, 253–257 (2009).
Elssner, T., Preusser, A., Wagner, U. & Kleber, H.P. Metabolism of l-carnitine by Enterobacteriaceae under aerobic conditions. FEMS Microbiol. Lett. 174, 295–301 (1999).
Möller, B., Hippe, H. & Gottschalk, G. Degradation of various amine compounds by mesophilic clostridia. Arch. Microbiol. 145, 85–90 (1986).
Eckburg, P.B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Kleber, H.P. Bacterial carnitine metabolism. FEMS Microbiol. Lett. 147, 1–9 (1997).
Hedayati, S.S. Dialysis-related carnitine disorder. Semin. Dial. 19, 323–328 (2006).
Mingorance, C., Rodriguez-Rodriguez, R., Justo, M.L., Alvarez de Sotomayor, M. & Herrera, M.D. Critical update for the clinical use of l-carnitine analogs in cardiometabolic disorders. Vasc. Health Risk Manag. 7, 169–176 (2011).
The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am. J. Epidemiol. 129, 687–702 (1989).
Chen, J. et al. Powerful statistical analysis for associating microbiomes to enviromental covariates using generalized Unifrac distances. Bioinformatics 28, 2106–2113 (2012).
Willems, G., Pison, G., Rousseeuw, P.J. & Van Aelst, S. A robust Hotelling test. Metrika 55, 125–138 (2002).
Acknowledgements
We thank L. Kerchenski and C. Stevenson for assistance in performing the clinical studies; A. Pratt, S. Neale, M. Pepoy and B. Sullivan for technical assistance with human specimen processing and routine clinical diagnostic testing; E. Klipfell, F. McNally and M. Berk for technical assistance; and the subjects who consented to participate in these studies. Mass spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX. Germ-free mice were obtained from the University of North Carolina Gnotobiotic Facility, which is supported by P30-DK034987-25-28 and P40-RR018603-06-08. This research was supported by US National Institutes of Health grants R01 HL103866 (S.L.H.), P20 HL113452 (S.L.H. and W.H.W.T.), PO1 HL30568 (A.J.L.), PO1 H28481 (A.J.L.), R00 HL096166 (J.M.B.), UH3-DK083981 (J.D.L.), 1RC1DK086472 (R.M.K.) and the Leducq Foundation (S.L.H.). The clinical study GeneBank was supported in part by P01 HL076491, P01 HL098055, R01 HL103931 and the Cleveland Clinic Foundation General Clinical Research Center of the Cleveland Clinic/Case Western Reserve University Clinical and Translational Science Award (1UL1RR024989). S.L.H. is also partially supported by a gift from the Leonard Krieger Fund. Z.W. was partially supported by a Scientist Development Grant from the American Heart Association. E.O. was supported by a MOBILITAS Postdoctoral Research Grant (MJD252). R.A.K. was supported in part by US National Institutes of Health grant T32 GM007250.
Author information
Authors and Affiliations
Contributions
R.A.K. participated in laboratory, mouse and human studies, assisted in statistical analyses, helped design the experiments and drafted the manuscript. Z.W. performed the initial metabolomics study and assisted with mouse and mass spectrometry analyses. B.S.L. synthesized d3- and d9-carnitine for studies, assisted with mass spectrometry analyses and helped draft the manuscript. E.B.B. and X.F. assisted in performance of mass spectrometry analyses of the large human clinical cohort study. Y.W. and L.L. performed the statistical analyses and critically reviewed the manuscript. J.D.S. helped with aortic root atherosclerosis analyses and critical review of the manuscript. J.A.D. assisted in experimental design. J.A.B. and B.T.S. assisted in laboratory and mouse experiments. E.O. and A.J.L. performed and helped interpret mouse cecal microbiota analyses. J.C., F.D.B., H.L., G.D.W., J.D.L. and R.M.K. assisted in human subject microbiota analyses and helped interpret human microbiota data. M.W. and J.M.B. assisted with measurement of bile acid pool size and helped with critical review of the manuscript. W.H.W.T. helped with human studies and critical review of the manuscript. S.L.H. conceived of the idea, helped design the experiments, provided the funding for the study and helped draft and critically revise the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.W. and B.S.L. are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and have the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposciences. W.H.W.T. received research grant support from Abbott Laboratories and served as a consultant for Medtronic and St. Jude Medical. S.L.H. and J.D.S. are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics patents. S.L.H. has been paid as a consultant or speaker by the following companies: Cleveland Heart Lab., Esperion, Liposciences, Merck & Co. and Pfizer. He has received research funds from Abbott, Cleveland Heart Lab., Esperion and Liposciences and has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories, Cleveland Heart Lab., Frantz Biomarkers, Liposciences and Siemens.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–23 and Supplementary Methods (PDF 2144 kb)
Rights and permissions
About this article
Cite this article
Koeth, R., Wang, Z., Levison, B. et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19, 576–585 (2013). https://doi.org/10.1038/nm.3145
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3145
This article is cited by
-
Plasma trimethylamine N-oxide metabolites in the second trimester predict the risk of hypertensive disorders of pregnancy: a nested case-control study
Hypertension Research (2024)
-
Heart Failure: a Punch from the Gut
Current Heart Failure Reports (2024)
-
Trimethylamine N-oxide promotes oxidative stress and lipid accumulation in macrophage foam cells via the Nrf2/ABCA1 pathway
Journal of Physiology and Biochemistry (2024)
-
Duodenal microbiome in chronic kidney disease
Clinical and Experimental Nephrology (2024)
-
Distinguishing benign and malignant thyroid nodules using plasma trimethylamine N-oxide, carnitine, choline and betaine
Journal of Cancer Research and Clinical Oncology (2024)