Abstract
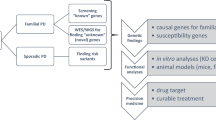
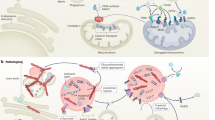
Recent years have seen an explosion in the rate of discovery of genetic defects linked to Parkinson's disease. These breakthroughs have not provided a direct explanation for the disease process. Nevertheless, they have helped transform Parkinson's disease research by providing tangible clues to the neurobiology of the disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dauer, W. & Przedborski, S. Parkinson's disease: mechanisms and models. Neuron 39, 889–909 (2003).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Spillantini, M.G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Polymeropoulos, M.H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 107–108 (1998).
Zarranz, J.J. et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 (2004).
Lo Bianco, C., Ridet, J.L., Schneider, B.L., Deglon, N. & Aebischer, P. α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 99, 10813–8 (2002).
Kirik, D. et al. Nigrostriatal α-synucleinopathy induced by viral vector-mediated overexpression of human α-synuclein: a new primate model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 100, 2884–2889 (2003).
Abeliovich, A. et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000).
Dauer, W. et al. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl. Acad. Sci. USA 99, 14524–14529 (2002).
Singleton, A.B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003).
Chartier-Harlin, M.C. et al. α-Synuclein locus duplication causes familial Parkinson's disease. Lancet (in the press).
Outeiro, T.F. & Lindquist, S. Yeast cells provide insight into α-synuclein biology and pathobiology. Science 302, 1772–1775 (2003).
Jao, C.C., Der-Sarkissian, A., Chen, J. & Langen, R. Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc. Natl. Acad. Sci. USA 101, 8331–8336 (2004).
Payton, J.E., Perrin, R.J., Woods, W.S. & George, J.M. Structural determinants of PLD2 inhibition by α-synuclein. J. Mol. Biol. 337, 1001–1009 (2004).
Xu, J. et al. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 8, 600–606 (2002).
Lee, H.J., Choi, C. & Lee, S.J. Membrane-bound α-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 277, 671–678 (2002).
Lee, H.J., Khoshaghideh, F., Patel, S. & Lee, S.J. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 24, 1888–1896 (2004).
Conway, K.A. et al. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 97, 571–576 (2000).
Volles, M.J. et al. Vesicle permeabilization by protofibrillar α-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry 40, 7812–7819 (2001).
Conway, K.A., Rochet, J.C., Bieganski, R.M. & Lansbury, P.T. Jr. Kinetic stabilization of the α-synuclein protofibril by a dopamine–α-synuclein adduct. Science 294, 1346–1349 (2001).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Goldberg, M.S. et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 278, 43628–43635 (2003).
Pesah, Y. et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131, 2183–2194 (2004).
Greene, J.C. et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 100, 4078–4083 (2003).
Palacino, J.J. et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 279, 18614–18622 (2004).
Shimura, H. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305 (2000).
Zhang, Y. et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. USA 97, 13354–13359 (2000).
Chung, K.K. et al. S-Nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304, 1328–1331 (2004).
Petrucelli, L. et al. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36, 1007–1019 (2002).
Staropoli, J.F. et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37, 735–749 (2003).
Leroy, E. et al. The ubiquitin pathway in Parkinson's disease. Nature 55, 512–521 (2004).
Maraganore, D.M. et al. UCHL1 is a Parkinson's disease susceptibility gene. Ann. Neurol. 55, 512–521 (2004).
Saigoh, K. et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat. Genet. 23, 47–51 (1999).
Liu, Y., Fallon, L., Lashuel, H.A., Liu, Z. & Lansbury, P.T. Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect α-synuclein degradation and Parkinson's disease susceptibility. Cell 111, 209–218 (2002).
Hemelaar, J. et al. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol. Cell Biol. 24, 84–95 (2004).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Hague, S. et al. Early-onset Parkinson's disease caused by a compound heterozygous DJ-1 mutation. Ann. Neurol. 54, 271–274 (2003).
Abou-Sleiman, P.M., Healy, D.G., Quinn, N., Lees, A.J. & Wood, N.W. The role of pathogenic DJ-1 mutations in Parkinson's disease. Ann. Neurol. 54, 283–286 (2003).
Cookson, M.R. Pathways to Parkinsonism. Neuron 37, 7–10 (2003).
Niki, T., Takahashi-Niki, K., Taira, T., Iguchi-Ariga, S.M. & Ariga, H. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. 1, 247–261 (2003).
Takahashi, K. et al. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx α to the receptor. J. Biol. Chem. 276, 37556–37563 (2001).
Taira, T. et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5, 213–218 (2004).
Quigley, P.M., Korotkov, K., Baneyx, F. & Hol, W.G. The 1.6-A crystal structure of the class of chaperones represented by Escherichia coli Hsp31 reveals a putative catalytic triad. Proc. Natl. Acad. Sci. USA 100, 3137–3142 (2003).
Moore, D.J., Zhang, L., Dawson, T.M. & Dawson, V.L. A missense mutation (L166P) in DJ-1, linked to familial Parkinson's disease, confers reduced protein stability and impairs homo-oligomerization. J. Neurochem. 87, 1558–1567 (2003).
Olzmann, J.A. et al. Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J. Biol. Chem. 279, 8506–8515 (2004).
Bandopadhyay, R. et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain 127, 420–430 (2004).
Valente, E.M. et al. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am. J. Hum. Genet. 68, 895–900 (2001).
Valente, E.M. et al. PARK6-linked parkinsonism occurs in several European families. Ann. Neurol. 51, 14–18 (2002).
Valente, E.M. et al. Hereditary early-onset parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 (2004).
Nakajima, A., Kataoka, K., Hong, M., Sakaguchi, M. & Huh, N.H. BRPK, a novel protein kinase showing increased expression in mouse cancer cell lines with higher metastatic potential. Cancer Lett. 201, 195–201 (2003).
Unoki, M. & Nakamura, Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 20, 4457–4465 (2001).
Khan, N.L. et al. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann. Neurol. 52, 849–853 (2002).
Farrer, M. et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 50, 293–300 (2001).
Acknowledgements
The authors thank R.E. Burke, D. Sulzer and W. Dauer for insightful comments on the manuscript, M.J. Farrer and A.B. Singleton for input concerning PARK4, and M. Lucas for assistance in preparing this manuscript. The authors are supported by NIH/NINDS (grants R29 NS37345, RO1 NS38586 and NS42269, P50 NS38370, and P01 NS11766-27A1), NIH/NIA grant (RO1 AG021617-01), the US Department of Defense (DAMD 17-99-1-9471, DAMD 17-03-1 and DAMD17-03-1-0428), the Lowenstein Foundation, the Lillian Goldman Charitable Trust, the Parkinson's Disease Foundation, the American Parkinson Disease Association, and MDA/Wings-Over-Wall Street.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vila, M., Przedborski, S. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med 10 (Suppl 7), S58–S62 (2004). https://doi.org/10.1038/nm1068
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1068
This article is cited by
-
IGF2 prevents dopaminergic neuronal loss and decreases intracellular alpha-synuclein accumulation in Parkinson’s disease models
Cell Death Discovery (2023)
-
Impact of infection on risk of Parkinson’s disease: a quantitative assessment of case-control and cohort studies
Journal of NeuroVirology (2019)
-
Low-Dose Aspirin Upregulates Tyrosine Hydroxylase and Increases Dopamine Production in Dopaminergic Neurons: Implications for Parkinson’s Disease
Journal of Neuroimmune Pharmacology (2019)
-
Modulating the catalytic activity of AMPK has neuroprotective effects against α-synuclein toxicity
Molecular Neurodegeneration (2017)
-
Neuromolecular imaging, a nanobiotechnology for Parkinson’s disease: advancing pharmacotherapy for personalized medicine
Journal of Neural Transmission (2017)