Abstract
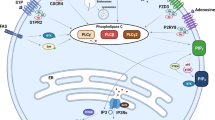
Ca2+ signals control a variety of lymphocyte responses, ranging from short-term cytoskeletal modifications to long-term changes in gene expression. The identification of molecules and channels that modulate Ca2+ entry into T and B lymphocytes has both provided details of the molecular events leading to immune responses and raised controversy. Here we review studies of the pathways that allow Ca2+ entry, the function of Ca2+ in the regulation of cell polarity and motility and the principles by which Ca2+-dependent transcription regulates lymphocyte function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bautista, D.M., Hoth, M. & Lewis, R.S. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J. Physiol. (Lond.) 541, 877–894 (2002).
Bautista, D.M. & Lewis, R.S. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. J. Physiol. (Lond.) 556, 805–817 (2004).
Hoth, M., Fanger, C.M. & Lewis, R.S. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol. 137, 633–648 (1997).
Hoth, M., Button, D.C. & Lewis, R.S. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl. Acad. Sci. USA 97, 10607–10612 (2000).
Makowska, A., Zablocki, K. & Duszynski, J. The role of mitochondria in the regulation of calcium influx into Jurkat cells. Eur. J. Biochem. 267, 877–884 (2000).
Lewis, R.S. & Cahalan, M.D. Potassium and calcium channels in lymphocytes. Annu. Rev. Immunol. 13, 623–653 (1995).
Berridge, M.J., Lipp, P. & Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Lewis, R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Randriamampita, C. & Trautmann, A. Ca2+ signals and T lymphocytes; new mechanisms and functions in Ca2+ signalling. Biol. Cell. 96, 69–78 (2004).
Huang, Y. & Wange, R.L. T cell receptor signaling: beyond complex complexes. J. Biol. Chem. 279, 28827–28830 (2004).
Patterson, R.L. et al. Phospholipase C-γ is required for agonist-induced Ca2+ entry. Cell 111, 529–541 (2002).
van Rossum, D.B. et al. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434, 99–104 (2005).
Yu, P. et al. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C γ-2 that specifically increases external Ca2+ entry. Immunity 22, 451–465 (2005).
Stork, B. et al. Grb2 and the non-T cell activation linker NTAL constitute a Ca2+-regulating signal circuit in B lymphocytes. Immunity 21, 681–691 (2004).
Singh, D.K. et al. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121, 281–293 (2005).
Brdicka, T. et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196, 1617–1626 (2002).
Janssen, E., Zhu, M., Zhang, W. & Koonpaew, S. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 4, 117–123 (2003).
Wang, Y. et al. Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol. Cell. Biol. 25, 4455–4465 (2005).
Volna, P. et al. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 200, 1001–1013 (2004).
Zhu, M., Liu, Y., Koonpaew, S., Granillo, O. & Zhang, W. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 200, 991–1000 (2004).
Janssen, E., Zhu, M., Craven, B. & Zhang, W. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-γ1 binding. J. Immunol. 172, 6810–6819 (2004).
Koonpaew, S., Janssen, E., Zhu, M. & Zhang, W. The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J. Biol. Chem. 279, 11229–11235 (2004).
Penninger, J.M. & Crabtree, G.R. The actin cytoskeleton and lymphocyte activation. Cell 96, 9–12 (1999).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Miletic, A.V., Swat, M., Fujikawa, K. & Swat, W. Cytoskeletal remodeling in lymphocyte activation. Curr. Opin. Immunol. 15, 261–268 (2003).
Huppa, J.B., Gleimer, M., Sumen, C. & Davis, M.M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 4, 749–755 (2003).
Lee, K.H. et al. T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Holsinger, L.J. et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Rivas, F.V., O'Keefe, J.P., Alegre, M.L. & Gajewski, T.F. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol. Cell. Biol. 24, 1628–1639 (2004).
Hao, S. & August, A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol. Biol. Cell 16, 2275–2284 (2005).
Mills, J.W., Falsig Pedersen, S., Walmod, P.S. & Hoffmann, E.K. Effect of cytochalasins on F-actin and morphology of Ehrlich ascites tumor cells. Exp. Cell Res. 261, 209–219 (2000).
Tybulewicz, V.L., Ardouin, L., Prisco, A. & Reynolds, L.F. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 (2003).
Gomez, M., Tybulewicz, V. & Cantrell, D.A. Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat. Immunol. 1, 348–352 (2000).
Zhang, J. et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 190, 1329–1342 (1999).
Walmsley, M.J. et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science 302, 459–462 (2003).
Tedford, K. et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2, 548–555 (2001).
Fischer, K.D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Snapper, S.B. et al. WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J. Leukoc. Biol. 77, 993–998 (2005).
Huang, W., Ochs, H.D., Dupont, B. & Vyas, Y.M. The Wiskott-Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-κB (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement. J. Immunol. 174, 2602–2611 (2005).
Cannon, J.L. & Burkhardt, J.K. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173, 1658–1662 (2004).
Silvin, C., Belisle, B. & Abo, A. A role for Wiskott-Aldrich syndrome protein in T-cell receptor-mediated transcriptional activation independent of actin polymerization. J. Biol. Chem. 276, 21450–21457 (2001).
Tybulewicz, V.L. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 17, 267–274 (2005).
Turner, M. B-cell development and antigen receptor signalling. Biochem. Soc. Trans. 30, 812–815 (2002).
Caloca, M.J., Zugaza, J.L., Matallanas, D., Crespo, P. & Bustelo, X.R. Vav mediates Ras stimulation by direct activation of the GDP/GTP exchange factor Ras GRP1. EMBO J. 22, 3326–3336 (2003).
Reynolds, L.F. et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 279, 18239–18246 (2004).
Cannon, J.L. & Burkhardt, J.K. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol. Rev. 186, 90–99 (2002).
Zeng, R. et al. SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. J. Immunol. 171, 1360–1368 (2003).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Higgs, H.N. & Pollard, T.D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676 (2001).
Gomez, T.S. et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 6, 261–270 (2005).
Hoth, M. & Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 (1992).
Premack, B.A., McDonald, T.V. & Gardner, P. Activation of Ca2+ current in Jurkat T cells following the depletion of Ca2+ stores by microsomal Ca2+-ATPase inhibitors. J. Immunol. 152, 5226–5240 (1994).
Zweifach, A. & Lewis, R.S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA 90, 6295–6299 (1993).
Takemura, H., Hughes, A.R., Thastrup, O. & Putney, J.W., Jr. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 264, 12266–12271 (1989).
Fanger, C.M., Hoth, M., Crabtree, G.R. & Lewis, R.S. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J. Cell Biol. 131, 655–667 (1995).
Serafini, A.T. et al. Isolation of mutant T lymphocytes with defects in capacitative calcium entry. Immunity 3, 239–250 (1995).
Feske, S., Giltnane, J., Dolmetsch, R., Staudt, L.M. & Rao, A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2, 316–324 (2001).
Liou, J. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005).
Roos, J. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005).
Zhang, S.L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005).
Ertel, E.A. et al. Nomenclature of voltage-gated calcium channels. Neuron 25, 533–535 (2000).
Genazzani, A.A., Carafoli, E. & Guerini, D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. USA 96, 5797–5801 (1999).
Graef, I.A. et al. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 401, 703–708 (1999).
Freedman, B.D., Price, M.A. & Deutsch, C.J. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J. Immunol. 149, 3784–3794 (1992).
Price, M., Lee, S.C. & Deutsch, C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA 86, 10171–10175 (1989).
Gomes, B. et al. Lymphocyte calcium signaling involves dihydropyridine-sensitive L-type calcium channels: facts and controversies. Crit. Rev. Immunol. 24, 425–447 (2004).
Chandy, K.G., DeCoursey, T.E., Cahalan, M.D., McLaughlin, C. & Gupta, S. Voltage-gated potassium channels are required for human T lymphocyte activation. J. Exp. Med. 160, 369–385 (1984).
Chandy, K.G. et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 25, 280–289 (2004).
Sadighi Akha, A.A. et al. Anti-Ig-induced calcium influx in rat B lymphocytes mediated by cGMP through a dihydropyridine-sensitive channel. J. Biol. Chem. 271, 7297–7300 (1996).
Grafton, G., Stokes, L., Toellner, K.M. & Gordon, J. A non-voltage-gated calcium channel with L-type characteristics activated by B cell receptor ligation. Biochem. Pharmacol. 66, 2001–2009 (2003).
Kotturi, M.F., Carlow, D.A., Lee, J.C., Ziltener, H.J. & Jefferies, W.A. Identification and functional characterization of voltage-dependent calcium channels in T lymphocytes. J. Biol. Chem. 278, 46949–46960 (2003).
Kotturi, M.F. & Jefferies, W.A. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol. Immunol. 42, 1461–1474 (2005).
Stokes, L., Gordon, J. & Grafton, G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major α pore-forming and auxiliary β-subunits. J. Biol. Chem. 279, 19566–19573 (2004).
Clapham, D.E., Runnels, L.W. & Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2, 387–396 (2001).
Gamberucci, A. et al. Diacylglycerol activates the influx of extracellular cations in T-lymphocytes independently of intracellular calcium-store depletion and possibly involving endogenous TRP6 gene products. Biochem. J. 364, 245–254 (2002).
Philipp, S. et al. TRPC3 mediates T-cell receptor-dependent calcium entry in human T-lymphocytes. J. Biol. Chem. 278, 26629–26638 (2003).
Liu, X., Bandyopadhyay, B.C., Singh, B.B., Groschner, K. & Ambudkar, I.S. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J. Biol. Chem. 280, 21600–21606 (2005).
Zagranichnaya, T.K., Wu, X. & Villereal, M.L. Endogenous TRPC1, TRPC3 and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 280, 29559–29569 (2005).
Lintschinger, B. et al. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 275, 27799–27805 (2000).
Hardie, R.C. Regulation of TRP channels via lipid second messengers. Annu. Rev. Physiol. 65, 735–759 (2003).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Venkatachalam, K., Zheng, F. & Gill, D.L. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 278, 29031–29040 (2003).
Yue, L., Peng, J.B., Hediger, M.A. & Clapham, D.E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410, 705–709 (2001).
Voets, T. et al. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J. Biol. Chem. 276, 47767–47770 (2001).
Murakami, M. et al. Identification and characterization of the murine TRPM4 channel. Biochem. Biophys. Res. Commun. 307, 522–528 (2003).
Launay, P. et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407 (2002).
Xu, X.Z., Moebius, F., Gill, D.L. & Montell, C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc. Natl. Acad. Sci. USA 98, 10692–10697 (2001).
Nilius, B. et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J. Biol. Chem. 278, 30813–30820 (2003).
Launay, P. et al. TRPM4 regulates calcium oscillations after T cell activation. Science 306, 1374–1377 (2004).
Nilius, B. et al. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 280, 6423–6433 (2005).
Goldsmith, M.A. & Weiss, A. Early signal transduction by the antigen receptor without commitment to T cell activation. Science 240, 1029–1031 (1988).
Delon, J., Bercovici, N., Liblau, R. & Trautmann, A. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur. J. Immunol. 28, 716–729 (1998).
Negulescu, P.A., Krasieva, T.B., Khan, A., Kerschbaum, H.H. & Cahalan, M.D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity 4, 421–430 (1996).
Wulfing, C. & Davis, M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Jacobelli, J., Chmura, S.A., Buxton, D.B., Davis, M.M. & Krummel, M.F. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat. Immunol. 5, 531–538 (2004).
Heath, K.E. et al. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am. J. Hum. Genet. 69, 1033–1045 (2001).
Conti, M.A., Even-Ram, S., Liu, C., Yamada, K.M. & Adelstein, R.S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 279, 41263–41266 (2004).
Matsushita, T. et al. Targeted disruption of mouse ortholog of the human MYH9 responsible for macrothrombocytopenia with different organ involvement: hematological, nephrological, and otological studies of heterozygous KO mice. Biochem. Biophys. Res. Commun. 325, 1163–1171 (2004).
Bhakta, N.R., Oh, D.Y. & Lewis, R.S. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat. Immunol. 6, 143–151 (2005).
Esser, M.T., Krishnamurthy, B. & Braciale, V.L. Distinct T cell receptor signaling requirements for perforin- or FasL-mediated cytotoxicity. J. Exp. Med. 183, 1697–1706 (1996).
Takayama, H. & Sitkovsky, M.V. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J. Exp. Med. 166, 725–743 (1987).
Kupfer, A., Dennert, G. & Singer, S.J. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J. Mol. Cell. Immunol. 2, 37–49 (1985).
Lowin-Kropf, B., Shapiro, V.S. & Weiss, A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell Biol. 140, 861–871 (1998).
Kuhn, J.R. & Poenie, M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16, 111–121 (2002).
Stinchcombe, J.C., Bossi, G., Booth, S. & Griffiths, G.M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Kuhne, M.R. et al. Linker for activation of T cells, ζ-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J. Immunol. 171, 860–866 (2003).
Lyubchenko, T.A., Wurth, G.A. & Zweifach, A. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity 15, 847–859 (2001).
Esser, M.T., Haverstick, D.M., Fuller, C.L., Gullo, C.A. & Braciale, V.L. Ca2+ signaling modulates cytolytic T lymphocyte effector functions. J. Exp. Med. 187, 1057–1067 (1998).
Purbhoo, M.A., Irvine, D.J., Huppa, J.B. & Davis, M.M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Thelen, M. Dancing to the tune of chemokines. Nat. Immunol. 2, 129–134 (2001).
Li, Z. et al. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science 287, 1046–1049 (2000).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2, 515–522 (2001).
Neptune, E.R. & Bourne, H.R. Receptors induce chemotaxis by releasing the βγ subunit of Gi, not by activating Gq or Gs . Proc. Natl. Acad. Sci. USA 94, 14489–14494 (1997).
Mandeville, J.T. & Maxfield, F.R. Effects of buffering intracellular free calcium on neutrophil migration through three-dimensional matrices. J. Cell. Physiol. 171, 168–178 (1997).
Eddy, R.J., Pierini, L.M., Matsumura, F. & Maxfield, F.R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298 (2000).
Spitaler, M. & Cantrell, D.A. Protein kinase C and beyond. Nat. Immunol. 5, 785–790 (2004).
Goldsmith, M.A., Desai, D.M., Schultz, T. & Weiss, A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J. Biol. Chem. 264, 17190–17197 (1989).
Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C. & Healy, J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 (1997).
Dolmetsch, R.E., Xu, K. & Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998).
Lewis, R.S. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem. Soc. Trans. 31, 925–929 (2003).
McKinsey, T.A., Zhang, C.L. & Olson, E.N. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97, 14400–14405 (2000).
Means, A.R. Regulatory cascades involving calmodulin-dependent protein kinases. Mol. Endocrinol. 14, 4–13 (2000).
McKinsey, T.A., Zhang, C.L., Lu, J. & Olson, E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 (2000).
Pan, F., Means, A.R. & Liu, J.O. Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation. EMBO J. 24, 2104–2113 (2005).
Youn, H.D. & Liu, J.O. Cabin1 represses MEF2-dependent Nur77 expression and T cell apoptosis by controlling association of histone deacetylases and acetylases with MEF2. Immunity 13, 85–94 (2000).
Meyer, T., Hanson, P.I., Stryer, L. & Schulman, H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science 256, 1199–1202 (1992).
Chow, F.A., Anderson, K.A., Noeldner, P.K. & Means, A.R. The autonomous activity of calcium/calmodulin-dependent protein kinase IV is required for its role in transcription. J. Biol. Chem. 280, 20530–20538 (2005).
Westphal, R.S., Anderson, K.A., Means, A.R. & Wadzinski, B.E. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science 280, 1258–1261 (1998).
Anderson, K.A., Noeldner, P.K., Reece, K., Wadzinski, B.E. & Means, A.R. Regulation and function of the calcium/calmodulin-dependent protein kinase IV/protein serine/threonine phosphatase 2A signaling complex. J. Biol. Chem. 279, 31708–31716 (2004).
Ishida, A., Kameshita, I. & Fujisawa, H. A novel protein phosphatase that dephosphorylates and regulates Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 273, 1904–1910 (1998).
Kitani, T. et al. Molecular cloning of Ca2+/calmodulin-dependent protein kinase phosphatase. J. Biochem. 125, 1022–1028 (1999).
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 (1991).
Emmel, E.A. et al. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science 246, 1617–1620 (1989).
Flanagan, W.M., Corthesy, B., Bram, R.J. & Crabtree, G.R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352, 803–807 (1991).
Clipstone, N.A. & Crabtree, G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357, 695–697 (1992).
Timmerman, L.A., Clipstone, N.A., Ho, S.N., Northrop, J.P. & Crabtree, G.R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383, 837–840 (1996).
Loh, C. et al. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J. Biol. Chem. 271, 10884–10891 (1996).
Beals, C.R., Sheridan, C.M., Turck, C.W., Gardner, P. & Crabtree, G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 (1997).
Sheridan, C.M., Heist, E.K., Beals, C.R., Crabtree, G.R. & Gardner, P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J. Biol. Chem. 277, 48664–48676 (2002).
Miskin, J.E., Abrams, C.C., Goatley, L.C. & Dixon, L.K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science 281, 562–565 (1998).
Gebert, B., Fischer, W., Weiss, E., Hoffmann, R. & Haas, R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301, 1099–1102 (2003).
Crabtree, G.R. & Olson, E.N. NFAT signaling: choreographing the social lives of cells. Cell 109, S67–S79 (2002).
Stankunas, K., Graef, I.A., Neilson, J.R., Park, S.H. & Crabtree, G.R. Signaling through calcium, calcineurin, and NF-AT in lymphocyte activation and development. Cold Spring Harb. Symp. Quant. Biol. 64, 505–516 (1999).
Rao, A., Luo, C. & Hogan, P.G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Crabtree, G.R. Contingent genetic regulatory events in T lymphocyte activation. Science 243, 355–361 (1989).
Diehn, M. et al. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc. Natl. Acad. Sci. USA 99, 11796–11801 (2002).
Parry, R.V. et al. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur. J. Immunol. 27, 2495–2501 (1997).
Harada, Y. et al. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J. Biol. Chem. 276, 9003–9008 (2001).
Jones, R.G. et al. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J. Exp. Med. 196, 335–348 (2002).
Kane, L.P., Andres, P.G., Howland, K.C., Abbas, A.K. & Weiss, A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-γ but not TH2 cytokines. Nat. Immunol. 2, 37–44 (2001).
Brunner, M.C. et al. CTLA-4-Mediated inhibition of early events of T cell proliferation. J. Immunol. 162, 5813–5820 (1999).
Chambers, C.A. et al. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol. Rev. 153, 27–46 (1996).
Gitler, A.D. et al. Nf1 has an essential role in endothelial cells. Nat. Genet. 33, 75–79 (2003).
Macian, F. et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 (2002).
Jenkins, M.K., Chen, C.A., Jung, G., Mueller, D.L. & Schwartz, R.H. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J. Immunol. 144, 16–22 (1990).
Jenkins, M.K., Pardoll, D.M., Mizuguchi, J., Chused, T.M. & Schwartz, R.H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc. Natl. Acad. Sci. USA 84, 5409–5413 (1987).
Heissmeyer, V. et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5, 255–265 (2004).
Mammucari, C. et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev. Cell 8, 665–676 (2005).
Kingsbury, T.J. & Cunningham, K.W. A conserved family of calcineurin regulators. Genes Dev. 14, 1595–1604 (2000).
Rothermel, B. et al. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 275, 8719–8725 (2000).
Lai, M.M., Burnett, P.E., Wolosker, H., Blackshaw, S. & Snyder, S.H. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 273, 18325–18331 (1998).
Sun, L. et al. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity 8, 703–711 (1998).
Fuentes, J.J. et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 9, 1681–1690 (2000).
Acknowledgements
We thank M. Winslow and J. Arron for critically reading the manuscript. Supported by a Stanford Graduate Fellowship (E.M.G.), Boehringer Ingelheim Fonds (K.C.-B.), the Howard Hughes Medical Institute (G.R.C.) and the National Institutes of Health (AI-60037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gallo, E., Canté-Barrett, K. & Crabtree, G. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol 7, 25–32 (2006). https://doi.org/10.1038/ni1295
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1295
This article is cited by
-
The crosstalk between cardiomyocyte calcium and inflammasome signaling pathways in atrial fibrillation
Pflügers Archiv - European Journal of Physiology (2021)
-
New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice
Clinical Reviews in Allergy & Immunology (2021)
-
TRP channels and traffic-related environmental pollution-induced pulmonary disease
Seminars in Immunopathology (2016)
-
Transient Receptor Potential (TRP) channels in T cells
Seminars in Immunopathology (2016)
-
A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication
Nature Medicine (2015)