Abstract
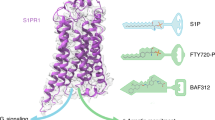
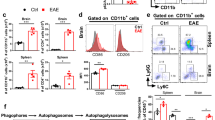
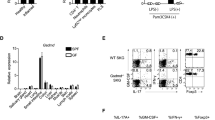
Sphingosine 1-phosphate (S1P) signaling regulates lymphocyte egress from lymphoid organs into systemic circulation. The sphingosine phosphate receptor 1 (S1P1) agonist FTY-720 (Gilenya) arrests immune trafficking and prevents multiple sclerosis (MS) relapses. However, alternative mechanisms of S1P-S1P1 signaling have been reported. Phosphoproteomic analysis of MS brain lesions revealed S1P1 phosphorylation on S351, a residue crucial for receptor internalization. Mutant mice harboring an S1pr1 gene encoding phosphorylation-deficient receptors (S1P1(S5A)) developed severe experimental autoimmune encephalomyelitis (EAE) due to autoimmunity mediated by interleukin 17 (IL-17)–producing helper T cells (TH17 cells) in the peripheral immune and nervous system. S1P1 directly activated the Jak-STAT3 signal-transduction pathway via IL-6. Impaired S1P1 phosphorylation enhances TH17 polarization and exacerbates autoimmune neuroinflammation. These mechanisms may be pathogenic in MS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baumruker, T., Billich, A. & Brinkmann, V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin. Investig. Drugs 16, 283–289 (2007).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Cohen, J.A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Devonshire, V. et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 11, 420–428 (2012).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Chi, H. & Flavell, R.A. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J. Immunol. 174, 2485–2488 (2005).
Rosen, H. & Goetzl, E.J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5, 560–570 (2005).
Cyster, J.G. & Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Zhi, L. et al. FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus. J. Immunol. 187, 2244–2251 (2011).
Rivera, J., Proia, R.L. & Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8, 753–763 (2008).
Allende, M.L. et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 (2004).
Halin, C. et al. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood 106, 1314–1322 (2005).
Oo, M.L. et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089 (2007).
Chun, J. & Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Ingwersen, J. et al. Fingolimod in multiple sclerosis: mechanisms of action and clinical efficacy. Clin. Immunol. 142, 15–24 (2012).
Cohen, J.A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Waubant, E. Emerging therapies for MS. Rev. Neurol. (Paris) 163, 688–696 (2007).
Kieseier, B.C., Wiendl, H., Hemmer, B. & Hartung, H.P. Treatment and treatment trials in multiple sclerosis. Curr. Opin. Neurol. 20, 286–293 (2007).
Jander, S., Turowski, B., Kieseier, B.C. & Hartung, H.P. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult. Scler. 18, 1650–1652 (2012).
Bourdette, D. & Gilden, D. Fingolimod and multiple sclerosis: four cautionary tales. Neurology 79, 1942–1943 (2012).
Visser, F., Wattjes, M.P., Pouwels, P.J., Linssen, W.H. & van Oosten, B.W. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology 79, 2000–2003 (2012).
Liu, C.H. et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 (1999).
Kohno, T. & Igarashi, Y. N-glycosylation of sphingosine 1-phosphate receptor, Edg-1, and its role on receptor internalization through membrane microdomain. Tanpakushitsu Kakusan Koso 47, 503–508 (2002).
Watterson, K.R. et al. Dual regulation of EDG1/S1P1 receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J. Biol. Chem. 277, 5767–5777 (2002).
Thangada, S. et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 207, 1475–1483 (2010).
Oo, M.L. et al. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121, 2290–2300 (2011).
Arnon, T.I. et al. GRK2-dependent S1P1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333, 1898–1903 (2011).
Han, M.H. et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 (2008).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Huttlin, E.L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Peng, J., Elias, J.E., Thoreen, C.C., Licklider, L.J. & Gygi, S.P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 (2003).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Huang, W., Sherman, B.T. & Lempicki, R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Krebs, E.G., Kent, A.B. & Fischer, E.H. The muscle phosphorylase b kinase reaction. J. Biol. Chem. 231, 73–83 (1958).
Mayya, V., Rezual, K., Wu, L., Fong, M.B. & Han, D.K. Absolute quantification of multisite phosphorylation by selective reaction monitoring mass spectrometry: determination of inhibitory phosphorylation status of cyclin-dependent kinases. Mol. Cell Proteomics 5, 1146–1157 (2006).
Chun, J. & Brinkmann, V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov. Med. 12, 213–228 (2011).
Hla, T. & Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 76, S3–S8 (2011).
Loh, K.C. et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE 7, e37218 (2012).
Rosen, H., Sanna, M.G., Cahalan, S.M. & Gonzalez-Cabrera, P.J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 28, 102–107 (2007).
Lovett-Racke, A.E., Yang, Y. & Racke, M.K. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim. Biophys. Acta 1812, 246–251 (2011).
Youssef, S. & Steinman, L. At once harmful and beneficial: the dual properties of NF-κB. Nat. Immunol. 7, 901–902 (2006).
Lee, M.J. et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279, 1552–1555 (1998).
Schwab, S.R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Allende, M.L., Dreier, J.L., Mandala, S. & Proia, R.L. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Zhou, J. & Saba, J.D. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 242, 502–507 (1998).
Lee, H. et al. STAT3-induced S1P1 expression is crucial for persistent STAT3 activation in tumors. Nat. Med. 16, 1421–1428 (2010).
Han, M.H. et al. Janus-like opposing roles of CD47 in autoimmune brain-inflammation in humans and mice. J. Exp. Med. 209, 1325–1334 (2012).
Arac, A. et al. Systemic augmentation of αB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc. Natl. Acad. Sci. USA 108, 13287–13292 (2011).
Acknowledgements
We thank J. Saba (Children's Hospital Oakland Research Institute) for providing THI. Supported by Neurology Department Startup Funds, Guthy-Jackson Charitable Foundation for Neuromyelitis Optica Research (M.H.H.), and US National Institutes of Health grants R37-HL67330, PO1-HL70694 and RO1HL89934 (T.H.).
Author information
Authors and Affiliations
Contributions
C.S.G. and M.H.H. formulated the hypothesis and designed all experiments. L.W., M.P.S. and D.K.H. contributed to the phosphoproteomic analysis. V.A.B. performed the in vitro experiment with S1P1-deficient T cells, and T.H. contributed to experiments related to S1P signaling. S.A. and D.B.L. assisted with siRNA experiments, R.A.S. with the histopathological studies, and A.A. and G.K.S. with intracellular cytokine staining and flow cytometry. R.C.A., P.P.H. and L.S. contributed to the EAE-related experiments. Y.H. and B.S.M. performed immunoblots and in vitro assays.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 2 and 3 (PDF 14246 kb)
Supplementary Table 1
Dataset from phosphoproteomic analysis of MS brain lesions. (XLSX 1188 kb)
Rights and permissions
About this article
Cite this article
Garris, C., Wu, L., Acharya, S. et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol 14, 1166–1172 (2013). https://doi.org/10.1038/ni.2730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2730
This article is cited by
-
Novel variants in CRB2 targeting the malfunction of slit diaphragm related to focal segmental glomerulosclerosis
Pediatric Nephrology (2024)
-
Functional roles of sphingolipids in immunity and their implication in disease
Experimental & Molecular Medicine (2023)
-
Fingolimod Alleviates Cognitive Deficit in Type 2 Diabetes by Promoting Microglial M2 Polarization via the pSTAT3-jmjd3 Axis
Molecular Neurobiology (2023)
-
Contribution of Intravital Neuroimaging to Study Animal Models of Multiple Sclerosis
Neurotherapeutics (2023)
-
S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission
Cellular & Molecular Immunology (2022)