Abstract
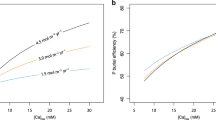
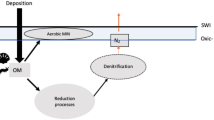
Animal burrowing and sediment-mixing (bioturbation) began during the run up to the Ediacaran/Cambrian boundary1,2,3, initiating a transition4,5 between the stratified Precambrian6 and more well-mixed Phanerozoic7 sedimentary records, against the backdrop of a variable8,9 global oxygen reservoir probably smaller in size than present10,11. Phosphorus is the long-term12 limiting nutrient for oxygen production via burial of organic carbon13, and its retention (relative to carbon) within organic matter in marine sediments is enhanced by bioturbation14,15,16,17,18. Here we explore the biogeochemical implications of a bioturbation-induced organic phosphorus sink in a simple model. We show that increased bioturbation robustly triggers a net decrease in the size of the global oxygen reservoir—the magnitude of which is contingent upon the prescribed difference in carbon to phosphorus ratios between bioturbated and laminated sediments. Bioturbation also reduces steady-state marine phosphate levels, but this effect is offset by the decline in iron-adsorbed phosphate burial that results from a decrease in oxygen concentrations. The introduction of oxygen-sensitive bioturbation to dynamical model runs is sufficient to trigger a negative feedback loop: the intensity of bioturbation is limited by the oxygen decrease it initially causes. The onset of this feedback is consistent with redox variations observed during the early Cambrian rise of bioturbation, leading us to suggest that bioturbation helped to regulate early oxygen and phosphorus cycles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liu, A. G., Mcllroy, D. & Brasier, M. D. First evidence for locomotion in the Ediacara biota from the 565 Ma Mistaken Point Formation, Newfoundland. Geology 38, 123–126 (2010).
Menon, L., McIlroy, D. & Brasier, M. D. Evidence for Cnidaria-like behavior in ca. 560 Ma Ediacaran Aspidella. Geology 41, 895–898 (2013).
Mangano, M & Buatois, L. A. Decoupling of body plan diversification and ecological structuring during the Ediacaran–Cambrian transition: Evolutionary and geobiological feedbacks. Proc. R. Soc. B 281, 20140038 (2014).
Buatois, L. A., Narbonne, G. M., Mangano, M. G., Carmona, M. B. & Myrow, P. Ediacaran matground persisted into the earliest Cambrian. Nature Commun. 5, 3544 (2014).
Tarhan, L. G. & Droser, M. L. Widespread delayed mixing in early to middle Cambrian marine shelfal settings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 399, 310–322 (2014).
Seilacher, A. Biomat-related lifestyles in the Precambrian. Palaios 14, 86–93 (1999).
Droser, M. L. & Bottjer, D. J. Trends and patterns of Phanerozoic ichnofabrics. Ann. Rev. Earth Planet. Sci. Lett. 21, 205–225 (1993).
Frei, R., Gaucher, C., Poulton, S. W. & Canfield, D. E. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461, 250–254 (2009).
Scott, C. et al. Tracing the stepwise oxidation of the Proterozoic ocean. Nature 452, 456–460 (2008).
Dahl, T. W. et al. Devonian rise in atmospheric oxygen correlated to the radiations in terrestrial plants and large predatory fish. Proc. Natl Acad. Sci. USA 107, 17911–17915 (2010).
Gill, B. C. et al. Geochemical evidence for widespread euxinia in the later Cambrian ocean. Nature 469, 80–83 (2011).
Redfield, A. C. The biological control of chemical factors in the environment. Am. Sci. 46, 205–221 (1958).
Betts, J. N. & Holland, H. D. The oxygen content of ocean bottom waters, the burial efficiency of organic carbon, and the regulation of atmospheric oxygen. Palaeogeogr. Palaeoclimatol. Palaeoecol. 97, 5–18 (1991).
Ingall, E. & Jahnke, R. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 58, 2571–2575 (1994).
Ingall, E. D., Bustin, R. M. & Van Cappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 57, 303–316 (1993).
Anderson, L. D., Delaney, M. L. & Faul, K. L. Carbon to phosphorus ratios in sediments: Implications for nutrient cycling. Glob. Biogeochem. Cycles 15, 65–79 (2001).
Aller, R. C. Bioturbation and remineralization of sedimentary organic matter: Effects of redox oscillation. Chem. Geol. 114, 331–345 (1994).
Kerrn-Jespersen, J. P. & Henze, M. Biological phosphorus uptake under anoxic and aerobic conditions. Water Res. 27, 617–624 (1993).
Droser, M. L. & Bottjer, D. J. Trends in depth and extent of bioturbation in Cambrian carbonate marine environments, western United States. Geology 16, 233–236 (1988).
McIlroy, D. & Logan, G. A. The impact of bioturbation on infaunal ecology and evolution during the Proterozoic–Cambrian transition. Palaios 14, 58–72 (1999).
Taylor, A., Goldring, R. & Gowland, S. Analysis and application of ichnofabrics. Earth Sci. Rev. 60, 227–259 (2003).
Saltzman, M. R. et al. Pulse of atmospheric oxygen during the late Cambrian. Proc. Natl Acad. Sci. USA 108, 3876–3881 (2011).
Berner, R. A. Burial of organic carbon and pyrite sulphur in the modern ocean: Its Geochemical and environmental significance. Am. J. Sci. 282, 451–473 (1982).
Slomp, C. P., Thomson, J. & de Lange, G. J. Controls on phosphorus regeneration and burial during formation of eastern Mediterranean sapropels. Mar. Geol. 203, 141–159 (2004).
Van Cappellen, P. & Ingall, E. D. Benthic phosphorus regeneration, net primary production, and ocean anoxia: A model of the coupled marine biogeochemical cycles of carbon and phosphorus. Paleoceanography 9, 677–692 (1994).
Lenton, T. M. & Watson, A. J. Redfield revisited: 2. What regulates the oxygen content of the atmosphere? Glob. Biogeochem. Cycles 14, 249–268 (2000).
Gundersen, J. K. & Jorgensen, B. B. Microstructure of diffusive boundary layers and the oxygen uptake of the seafloor. Nature 345, 604–607 (1993).
Papineau, D. Global biogeochemical changes at both ends of the proterozoic: Insights from phosphorites. Astrobiology 10, 165–181 (2010).
Algeo, T. J. & Ingall, E. Sedimentary Corg:P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2 . Palaeogeogr. Palaeoclimatol. Palaeoecol. 256, 130–155 (2007).
Partin, C. A. et al. Large scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales. Earth. Plan. Sci. Lett. 369–370, 284–293 (2013).
Acknowledgements
R.A.B., T.M.L. and G.A.S-Z. gratefully acknowledge funding from the National Environment Research Council (NE/I005978/1). T.W.D. was sponsored from the Inge Lehmann Scholarship and the VILLUM Foundation (VKR023127). M.Z. is funded by the National Basic Research Program of China (2013CB835000) and the National Natural Science Foundation of China (40930211). A.W.D. was supported by the SFB754, funded by the German DFG (www.sfb754.de).
Author information
Authors and Affiliations
Contributions
R.A.B., T.M.L., G.A.S-Z. and M.Z. developed the hypothesis, including ideas from all co-authors. T.W.D. provided data. R.A.B. modified the original model of T.M.L. R.A.B. wrote the paper with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2234 kb)
Supplementary Information
Supplementary Information (XLSX 48 kb)
Rights and permissions
About this article
Cite this article
Boyle, R., Dahl, T., Dale, A. et al. Stabilization of the coupled oxygen and phosphorus cycles by the evolution of bioturbation. Nature Geosci 7, 671–676 (2014). https://doi.org/10.1038/ngeo2213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2213
This article is cited by
-
Jurassic paleosurfaces with fecal mounds reveal the last supper of arenicolid worms
Scientific Reports (2024)
-
Neogene burial of organic carbon in the global ocean
Nature (2023)
-
Increased habitat segregation at the dawn of the Phanerozoic revealed by correspondence analysis of bioturbation
Scientific Reports (2023)
-
Biological matter enhanced iron release from shallow marine bioturbated sediments: a case study of Late Cretaceous sandstone, northern Saudi Arabia
International Journal of Earth Sciences (2023)
-
Bioturbation of Thalassinoides from the Lower Cambrian Zhushadong Formation of Dengfeng area, Henan Province, North China
Journal of Palaeogeography (2021)