Abstract
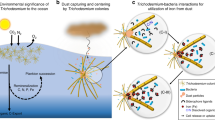
Trichodesmium, a filamentous dinitrogen-fixing cyanobacterium, forms extensive blooms in nutrient-poor tropical and subtropical ocean waters. These cyano-bacteria contribute significantly to biological fixation of nitrogen from the atmosphere in these waters, and thereby fuel primary production and influence nutrient flow and the cycling of organic and inorganic matter1,2. Trichodesmium blooms require large quantities of iron, which is partly supplied by the influx of wind-blown dust3. However, the processes and mechanisms associated with dust acquisition are poorly understood3,4,5,6. Here, we incubate natural populations and laboratory cultures of Trichodesmium with isotopically labelled iron oxides and desert dust, to determine how these cyanobacteria collect, process and use particulate iron. We show that, like most phytoplankton, Trichodesmium acquires only dissolved iron. However, unlike other studied phytoplankton, Trichodesmium accelerates the rate of iron dissolution from oxides and dust, through as yet unspecified cell-surface processes, and thereby increases cellular iron uptake rates. We show that natural puff (ball-shaped) colonies of Trichodesmium are particularly effective at dissolving dust and oxides, which we attribute to efficient dust trapping in their intricate colony morphology, followed by active shuttling and packaging of the dust within the colony core. We suggest that colony formation in Trichodesmium is an adaptive strategy that enhances iron acquisition from particulate sources such as dust.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carpenter, E. J. & Capone, D. G. in Nitrogen in the Marine Environment 2nd edn (eds Capone, D. G., Bronk, D. A., Mulholland, M. & Carpenter, E. J.) 141–198 (Elsevier, 2008).
LaRoche, J. & Breitbarth, E. Importance of the diazotrophs as a source of new nitrogen in the ocean. J. Sea Res. 53, 67–91 (2005).
Boyd, P. W. & Elwood, M. J. The biogeochemical cycle of iron in the ocean. Nature Geosci. 3, 675–682 (2010).
Boyd, P. W., Mackie, D. S. & Hunter, K. A. Aerosol iron deposition to the surface ocean—modes of iron supply and biological responses. Mar. Chem. 120, 128–143 (2010).
Baker, A. R. & Croot, P. L. Atmospheric and marine controls on aerosol iron solubility in seawater. Mar. Chem. 120, 4–13 (2010).
Breitbarth, E. et al. Iron biogeochemistry across marine systems—progress from the past decade. Biogeosciences 7, 1075–1097 (2010).
Darwin, C. R. Journal of Researches into the Geology and Natural History of the Various Countries Visited by H.M.S. Beagle, Under the Command of Captain Fitzroy, R.N. from 1832 to 1836. (Henry Colburn, 1839).
Capone, D. G., Zehr, J. P., Paerl, H. W., Bergman, B. & Carpenter, E. J. Trichodesmium, a globally significant marine cyanobacterium. Science 276, 1221–1229 (1997).
Paerl, H. W. in Growth and Reproductive Strategies of Freshwater Phytoplankton (ed. Sandgren, C. D.) 261–315 (Cambridge Univ. Press, 1988).
Villareal, T. A. & Carpenter, E. J. Diel buoyancy regulation in the marine diazotrophic cyanobacterium Trichodesmium thiebautii. Limnol. Oceanogr. 35, 1832–1837 (1990).
Paerl, H. W. & Bebout, B. M. in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs (eds Carpenter, E. J., Capone, D. G. & Rueter, J. G.) 43–60 (Kluwer Academic, 1992).
Sunda, W. G. in The Biogeochemistry of Iron in Seawater (eds Hunter, K. A. & Turner, D. R.) 41–84 (Wiley, 2001).
Rueter, J. G., Hutchins, D. A., Smith, R. W. & Unsworth, N. L. in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs (eds Carpenter, E. J., Capone, D. G. & Rueter, J. G.) 289–306 (Kluwer Academic, 1992).
Berman-Frank, I., Cullen, J. T., Shaked, Y., Sherrell, R. M. & Falkowski, P. G. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 46, 1249–1260 (2001).
Moore, M. C. et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nature Geosci. 2, 867–871 (2009).
Lenes, J. M. et al. Saharan dust and phosphatic fidelity: A three-dimensional biogeochemical model of Trichodesmium as a nutrient source for red tides on the West Florida Shelf. Cont. Shelf Res. 28, 1091–1115 (2008).
Fernández, A., Mouriño-Carballido, B., Bode, A., Varela, M. & Marañón, E. Latitudinal distribution of Trichodesmium spp. and N2 fixation in the Atlantic Ocean. Biogeosciences 7, 3167–3176 (2010).
Rich, H. W. & Morel, F. M. M. Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 35, 652–662 (1990).
Rose, A. L. & Waite, T. D. Kinetics of hydrolysis and precipitation of ferric iron in seawater. Environ. Sci. Technol. 37, 3897–3903 (2003).
Castruita, M., Elmegreen, L. A., Shaked, Y., Stiefel, E. I. & Morel, F. M. M. Comparison of the kinetics of iron release from a marine (Trichodesmium erythraeum) Dps protein and mammalian ferritin in the presence and absence of ligands. J. Inor. Biochem. 101, 1686–1691 (2007).
Halfen, L. N. & Castenholz, R. W. Gliding motility in the blue–green alga Oscillatoria princeps. J. Phycol. 7, 133–145 (1971).
Pate, J. & Chang, L-Y. Evidence that gliding motility in prokaryotic cells is driven by rotary assemblies in the cell envelopes. Curr. Microbiol. 2, 59–64 (1979).
Carlton, R. G. & Paerl, H. W. Oxygen-induces changes in morphology of aggregates of Aphanizomenon flos-aquae (Cyanophyceae): Implications for nitrogen fixation potentials. J. Phycol. 25, 326–333 (1989).
Kraemer, S. M., Butler, A., Borer, P. & Cervini-Silva, J. Siderophores and the dissolution of iron-bearing minerals in marine systems. Rev. Mineral. Geochem. 59, 53–84 (2005).
Shaked, Y., Kustka, A. B. & Morel, F. M. M. A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol. Oceanogr. 50, 872–882 (2005).
Barbeau, K. Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem. Photobiol. 82, 1505–1516 (2006).
Chen, Y. B., Zehr, J. P. & Mellon, M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: Evidence for a circadian rhythm. J. Phycol. 32, 916–923 (1996).
Erel, Y., Dayan, U., Rabi, R., Rudich, Y. & Stein, M. Trans boundary transport of pollutants by atmospheric mineral dust. Environ. Sci. Technol. 40, 2996–3005 (2006).
Hudson, R. J. M. & Morel, F. M. M. Distinguishing between extracellular and intracellular iron in marine phytoplankton. Limnol. Oceanogr. 34, 1113–1120 (1989).
Acknowledgements
We wish to thank O. Levitan and D. Spungin for help with Trichodesmium culturing, H. Lis for help with uptake experiments, and V. Farstey, M. Dray and I. Avishay for help with natural Trichodesmium collection. This work was supported in part by the Israel Science Foundation grant 933/07 and the Israel USA Binational Science Foundation grant 2008097 awarded to Y.S. and BMBF-MOST GR1950 to I.B-F. This work is in partial fulfilment of the requirements for a MSc thesis to M.R. from Bar Ilan University.
Author information
Authors and Affiliations
Contributions
The work presented in this paper is a joint effort of all authors. Y.S. conceived the study; Y.S., I.B-F. and M.R. planned and designed the experiments; M.R. carried out the experiments and analysed the data; all authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 517 kb)
Supplementary Movies
Supplementary Information (WMV 10383 kb)
Supplementary Movies
Supplementary Information (WMV 7095 kb)
Rights and permissions
About this article
Cite this article
Rubin, M., Berman-Frank, I. & Shaked, Y. Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nature Geosci 4, 529–534 (2011). https://doi.org/10.1038/ngeo1181
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1181
This article is cited by
-
Iron uptake, transport and storage in marine brown algae
BioMetals (2023)
-
Biological matter enhanced iron release from shallow marine bioturbated sediments: a case study of Late Cretaceous sandstone, northern Saudi Arabia
International Journal of Earth Sciences (2023)
-
Phycosphere pH of unicellular nano- and micro- phytoplankton cells and consequences for iron speciation
The ISME Journal (2022)
-
Assessing the contribution of diazotrophs to microbial Fe uptake using a group specific approach in the Western Tropical South Pacific Ocean
ISME Communications (2022)
-
Mechanisms and heterogeneity of in situ mineral processing by the marine nitrogen fixer Trichodesmium revealed by single-colony metaproteomics
ISME Communications (2021)