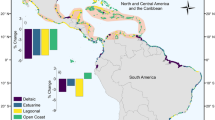
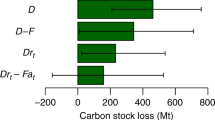
Abstract
Mangrove forests occur along ocean coastlines throughout the tropics, and support numerous ecosystem services, including fisheries production and nutrient cycling. However, the areal extent of mangrove forests has declined by 30–50% over the past half century as a result of coastal development, aquaculture expansion and over-harvesting1,2,3,4. Carbon emissions resulting from mangrove loss are uncertain, owing in part to a lack of broad-scale data on the amount of carbon stored in these ecosystems, particularly below ground5. Here, we quantified whole-ecosystem carbon storage by measuring tree and dead wood biomass, soil carbon content, and soil depth in 25 mangrove forests across a broad area of the Indo-Pacific region—spanning 30° of latitude and 73° of longitude—where mangrove area and diversity are greatest4,6. These data indicate that mangroves are among the most carbon-rich forests in the tropics, containing on average 1,023 Mg carbon per hectare. Organic-rich soils ranged from 0.5 m to more than 3 m in depth and accounted for 49–98% of carbon storage in these systems. Combining our data with other published information, we estimate that mangrove deforestation generates emissions of 0.02–0.12 Pg carbon per year—as much as around 10% of emissions from deforestation globally, despite accounting for just 0.7% of tropical forest area6,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Duke, N. C. et al. A world without mangroves? Science 317, 41–42 (2007).
Polidoro, B. A. et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 5, e10095 (2010).
Alongi, D. M. Present state and future of the world’s mangrove forests. Environ. Conserv. 29, 331–349 (2002).
Food and Agriculture Organization of the United Nations (FAO). The World’s Mangroves 1980–2005 (FAO Forestry Paper 153. FAO, 2007).
Bouillon, S., Rivera-Monroy, V. H., Twilley, R. R., Kairo, J. G. & Mangroves, in The Management of Natural Coastal Carbon Sinks (eds Laffoley, D. & Grimsditch) (IUCN, 2009).
Giri, C. et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 20, 154–159 (2011).
van der Werf, G. R. et al. CO2 emissions from forest loss. Nature Geosci. 2, 737–738 (2009).
IPCC in The Fourth Assessment Report Climate Change 2007 (eds Pachauri, R. K. & Reisinger, A.) (IPCC, 2007).
Intergovernmental Panel on Climate Change (IPCC) in Good Practice Guidance for Land use, Land-use Change, and Forestry (eds Penman, J. et al.) (Institute for Global Environmental Strategies, 2003).
Keith, H., Mackey, B. G. & Lindenmayer, D. B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl Acad. Sci. USA 106, 11635–11640 (2009).
Page, S. E. et al. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420, 61–65 (2002).
Page, S. E., Rieley, J. O. & Banks, C. J. Global and regional importance of the tropical peatland carbon pool. Glob. Change Biol. 17, 798–818 (2011).
Murdiyarso, D. M., Hergoualc’h, K. & Verchot, L. V. Opportunities for reducing greenhouse gas emissions in tropical peatlands. Proc. Natl Acad. Sci. USA 107, 19655–19660 (2010).
Gilman, E. L., Ellison, J., Duke, N. C. & Field, C. Threats to mangroves from climate change and adaptation options. Aquat. Bot. 89, 237–250 (2008).
Alongi, D. M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast Shelf Sci. 76, 1–13 (2008).
Kristensen, E., Bouillon, S., Dittmar, T. & Marchand, C. Organic carbon dynamics in mangrove ecosystems. Aquat. Bot. 89, 201–219 (2008).
Komiyama, A., Ong, J. E. & Poungparn, S. Allometry, biomass, and productivity of mangrove forests. Aquat. Bot. 89, 128–137 (2008).
Twilley, R. R., Chen, R. H. & Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut. 64, 265–288 (1992).
Bouillon, S. et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 22, GB2013 (2008).
Alongi, D. M. et al. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land–ocean–atmosphere exchange in peninsular Malaysia. Mar. Geol. 208, 383–402 (2004).
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R. & Lynch, J. C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 17, 1111 (2003).
Eong, O. J. Mangroves—a carbon source and sink. Chemosphere 27, 1097–1107 (1993).
Golley, F., Odum, H. T. & Wilson, R. F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 43, 9–19 (1962).
Fujimoto, K. et al. Belowground carbon storage of Micronesian mangrove forests. Ecol. Res. 14, 409–413 (1999).
Matsui, N. Estimated stocks of organic carbon in mangrove roots and sediments in Hinchinbrook Channel, Australia. Mangr. Salt Marsh. 2, 199–204 (1998).
Sjöling, S., Mohammed, S. M., Lyimo, T. J. & Kyaruzi, J. J. Benthic bacterial diversity and nutrient processes in mangroves: Impact of deforestation. Estuar. Coast Shelf Sci. 63, 397–406 (2005).
Strangmann, A., Bashan, Y. & Giani, L. Methane in pristine and impaired mangrove soils and its possible effects on establishment of mangrove seedlings. Biol. Fertil. Soils 44, 511–519 (2008).
Granek, E. & Ruttenberg, B. I. Changes in biotic and abiotic processes following mangrove clearing. Estuar. Coast Shelf Sci. 80, 555–562 (2008).
Hooijer, A., Silvius, M., Wösten, H. & Page, S. PEAT-CO2: Assessment of CO 2 Emissions from Drained Peatlands in SE Asia. Delft Hydraulics Report Q3943 (2006).
Church, J. A. et al. Understanding global sea levels: Past, present and future. Sustain. Sci. 3, 9–22 (2008).
Acknowledgements
We thank our many international partners and field personnel for assistance with logistics and data collection: Kosrae Island Resource Management Authority; Yap State Forestry; Orangutan Foundation International; Indonesian Directorate General for Forest Protection and Nature Conservation; University of Manado and Bogor Agricultural University, Indonesia; Bangladesh Forest Department; and KPSKSA (Cilacap, Indonesia). We thank K. Gerow for statistical assistance, and R. Mackenzie, C. Kryss and J. Bonham for assistance compiling site data. Funding was provided by USDA Forest Service International Programs and the Australian Agency for International Development (AusAID).
Author information
Authors and Affiliations
Contributions
D.C.D. co-designed the study, collected field data, performed data analyses, and led the writing of the paper. J.B.K. conceived and co-designed the study, and contributed to data collection and writing. D.M. co-conceived the study, arranged for and contributed to data collection, and contributed to writing. S.K. contributed to data collection, data analysis, and writing. M.S. collected field data and contributed to writing. M.K. co-conceived the study and contributed to writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 352 kb)
Rights and permissions
About this article
Cite this article
Donato, D., Kauffman, J., Murdiyarso, D. et al. Mangroves among the most carbon-rich forests in the tropics. Nature Geosci 4, 293–297 (2011). https://doi.org/10.1038/ngeo1123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1123
This article is cited by
-
Stronger increases but greater variability in global mangrove productivity compared to that of adjacent terrestrial forests
Nature Ecology & Evolution (2024)
-
Warming and flooding have different effects on organic carbon stability in mangrove soils
Journal of Soils and Sediments (2024)
-
Determining Environmental Drivers of Fine-Scale Variability in Blue Carbon Soil Stocks
Estuaries and Coasts (2024)
-
Microplastics modify the microbial-mediated carbon metabolism in mangroves
Environmental Chemistry Letters (2024)
-
Microcosm study on fate and dynamics of mangrove tannins during leaf litter leaching
Ecological Processes (2023)