Abstract
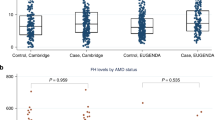
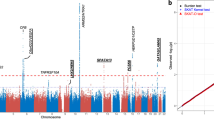
Up to half of the heritability of age-related macular degeneration (AMD) is explained by common variants1,2,3,4,5. Here, we report the identification of a rare, highly penetrant missense mutation in CFI encoding a p.Gly119Arg substitution that confers high risk of AMD (P = 3.79 × 10−6; odds ratio (OR) = 22.20, 95% confidence interval (CI) = 2.98–164.49). Plasma and sera from cases carrying the p.Gly119Arg substitution mediated the degradation of C3b, both in the fluid phase and on the cell surface, to a lesser extent than those from controls. Recombinant protein studies showed that the Gly119Arg mutant protein is both expressed and secreted at lower levels than wild-type protein. Consistent with these findings, human CFI mRNA encoding Arg119 had reduced activity compared to wild-type mRNA encoding Gly119 in regulating vessel thickness and branching in the zebrafish retina. Taken together, these findings demonstrate that rare, highly penetrant mutations contribute to the genetic burden of AMD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Despriet, D.D., Klaver, C.C., van Duijn, C.C. & Janssens, A.C. Predictive value of multiple genetic testing for age-related macular degeneration. Arch. Ophthalmol. 125, 1270–1271 (2007).
Maller, J. et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat. Genet. 38, 1055–1059 (2006).
Manolio, T.A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Seddon, J.M. et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest. Ophthalmol. Vis. Sci. 50, 2044–2053 (2009).
Yu, Y. et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum. Mol. Genet. 20, 3699–3709 (2011).
Friedman, D.S. et al. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564–572 (2004).
Vingerling, J.R., Klaver, C.C., Hofman, A. & de Jong, P.T. Epidemiology of age-related maculopathy. Epidemiol. Rev. 17, 347–360 (1995).
Chen, W. et al. Genetic variants near TIMP3 and high-density lipoprotein–associated loci influence susceptibility to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 107, 7401–7406 (2010).
Klein, R.J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389 (2005).
Neale, B.M. et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. USA 107, 7395–7400 (2010).
Boon, C.J.F. et al. Basal laminar drusen caused by compound heterozygous variants in the CFH gene. Am. J. Hum. Genet. 82, 516–523 (2008).
Raychaudhuri, S. et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 43, 1232–1236 (2011).
Schultz, D.W. et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum. Mol. Genet. 12, 3315–3323 (2003).
van de Ven, J.P.H. et al. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch. Ophthalmol. 130, 1038–1047 (2012).
Nilsson, S.C., Sim, R.B., Lea, S.M., Fremeaux-Bacchi, V. & Blom, A.M. Complement factor I in health and disease. Mol. Immunol. 48, 1611–1620 (2011).
Roversi, P. et al. Structural basis for complement factor I control and its disease-associated sequence polymorphisms. Proc. Natl. Acad. Sci. USA 108, 12839–12844 (2011).
Bienaime, F. et al. Mutations in components of complement influence the outcome of Factor I–associated atypical hemolytic uremic syndrome. Kidney Int. 77, 339–349 (2010).
Nilsson, S.C. et al. Genetic, molecular and functional analyses of complement factor I deficiency. Eur. J. Immunol. 39, 310–323 (2009).
Wang, J. et al. Altered function of factor I caused by amyloid β: implication for pathogenesis of age-related macular degeneration from Drusen. J. Immunol. 181, 712–720 (2008).
Fagerness, J.A. et al. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genet. 17, 100–104 (2009).
Hohenester, E., Sasaki, T. & Timpl, R. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat. Struct. Biol. 6, 228–232 (1999).
Hooft, R.W., Sander, C. & Vriend, G. Objectively judging the quality of a protein structure from a Ramachandran plot. Comput. Appl. Biosci. 13, 425–430 (1997).
Kleywegt, G.J. & Jones, T.A. Phi/psi-chology: Ramachandran revisited. Structure 4, 1395–1400 (1996).
Isogai, S., Horiguchi, M. & Weinstein, B.M. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 230, 278–301 (2001).
Nasevicius, A., Larson, J. & Ekker, S.C. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast 17, 294–301 (2000).
Bahary, N. et al. Duplicate VegfA genes and orthologues of the KDR receptor tyrosine kinase family mediate vascular development in the zebrafish. Blood 110, 3627–3636 (2007).
Zeng, X.X. et al. Phospholipase D1 is required for angiogenesis of intersegmental blood vessels in zebrafish. Dev. Biol. 328, 363–376 (2009).
Maga, T.K., Nishimura, C.J., Weaver, A.E., Frees, K.L. & Smith, R.J. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum. Mutat. 31, E1445–E1460 (2010).
Weiner, D.E., Tighiouart, H., Reynolds, R. & Seddon, J.M. Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol. Dial. Transplant. 26, 3159–3165 (2011).
Fakhouri, F. et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J. Am. Soc. Nephrol. 21, 859–867 (2010).
Sobrin, L. et al. Genetic profile for five common variants associated with age-related macular degeneration in densely affected families: a novel analytic approach. Eur. J. Hum. Genet. 18, 496–501 (2010).
van Leeuwen, R. et al. Grading of age-related maculopathy for epidemiological studies: is digital imaging as good as 35-mm film? Ophthalmology 110, 1540–1544 (2003).
Fauser, S. et al. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 5525–5528 (2011).
Smailhodzic, D. et al. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology 119, 339–346 (2012).
Ho, L. et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch. Ophthalmol. 129, 758–766 (2011).
Yang, Z. et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N. Engl. J. Med. 359, 1456–1463 (2008).
Yang, Z. et al. Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 6, e1000836 (2010).
Dahlbäck, B. Purification of human C4b-binding protein and formation of its complex with vitamin K–dependent protein S. Biochem. J. 209, 847–856 (1983).
Blom, A.M., Kask, L. & Dahlback, B. CCP1–4 of the C4b-binding protein α-chain are required for factor I mediated cleavage of complement factor C3b. Mol. Immunol. 39, 547–556 (2003).
Nilsson, S.C. et al. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Mol. Immunol. 44, 1835–1844 (2007).
Krieger, E., Koraimann, G. & Vriend, G. Increasing the precision of comparative models with YASARA NOVA—a self-parameterizing force field. Proteins 47, 393–402 (2002).
Levey, A.S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999).
Acknowledgements
We thank B. Janssen, A. Brücker, T. Janssen-van Kempen and M. Verbiest for excellent technical assistance. This study was supported by the Netherlands Organization for Scientific Research (Vidi Innovational Research Award 016.096.309 to A.I.d.H.), the Foundation Fighting Blindness (grant C-GE-0811-0548-RAD04 to A.I.d.H.), the Swedish Research Council (K2012-66X-14928-09-5 to A.M.B.) and the Söderberg Foundation (to A.M.B.).
Author information
Authors and Affiliations
Contributions
J.P.H.v.d.V., S.C.N., P.L.T., S.B.N., N.K., A.M.B. and A.I.d.H. wrote the manuscript, and all authors approved and commented on it. J.P.H.v.d.V., G.H.S.B., T.R., D.S., P.A.C., D.J.Z., C.J.F.B., S.F., S.L., B.J.K., M.R. Duvvari, N.K., C.C.W.K. and C.B.H. recruited and evaluated subjects. J.P.H.v.d.V., P.L.T., F.E.S.-K., M.R. Daha, A.G.U., B.B. and C.C.P. performed the genetic analyses. S.C.N., F.C.M. and A.M.B. performed the functional FI assays. S.B.N. performed the structural analysis of the FI variants. P.L.T. and N.K. performed the in vivo analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–6 (PDF 119 kb)
Rights and permissions
About this article
Cite this article
van de Ven, J., Nilsson, S., Tan, P. et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet 45, 813–817 (2013). https://doi.org/10.1038/ng.2640
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2640
This article is cited by
-
Knockdown of myorg leads to brain calcification in zebrafish
Molecular Brain (2022)
-
C1q and the classical complement cascade in geographic atrophy secondary to age-related macular degeneration
International Journal of Retina and Vitreous (2022)
-
Age-related macular degeneration and premorbid allergic diseases: a population-based case–control study
Scientific Reports (2021)
-
A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration
Nature Communications (2019)
-
Association of polymorphisms of complement factor I rs141853578 (G119R) with age-related macular degeneration in Iranian population
International Ophthalmology (2019)