Abstract
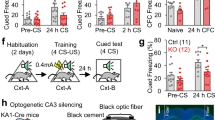
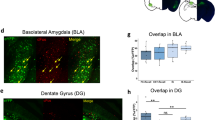
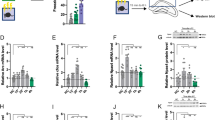
Memory reconsolidation has been demonstrated in various tasks and species, suggesting it is a fundamental process. However, there are experimental parameters that can inhibit reconsolidation from occurring (boundary conditions). These conditions and their mechanisms remain poorly defined. Here, we characterize the ability of strong training to inhibit reconsolidation at the behavioral, systems and molecular levels. We demonstrate that strong memories in rats initially are resistant to reconsolidation, but after sufficient time will undergo reconsolidation, suggesting that boundary conditions can be transient. At the systems level, we show that the hippocampus is necessary for inhibiting reconsolidation in the amygdala. At the molecular level, we demonstrate that NR2B NMDA-receptor subunits which are critical for the induction of reconsolidation of auditory memories in the amygdala, are downregulated only under conditions when strong memories do not undergo reconsolidation. This suggests that one molecular mechanism for mediating boundary conditions is through downregulation of reconsolidation induction mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Müller, G.E. & Pilzecker, A. Experimentelle Beitrage zur Lehre vom Gedachtnis [Experimental contributions to the theory of memory]. Z. Psychol. Erganzungsband (Z. Psychol. suppl.) 1 1:300 (1900).
Misanin, J.R., Miller, R.R. & Lewis, D.J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160, 554–555 (1968).
Schneider, A.M. & Sherman, W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science 159, 219–221 (1968).
Alberini, C.M. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56 (2005).
Nader, K., Schafe, G.E. & LeDoux, J.E. The labile nature of consolidation theory. Nat. Rev. Neurosci. 1, 216–219 (2000).
Dudai, Y. Reconsolidation: the advantage of being refocused. Curr. Opin. Neurobiol. 16, 174–178 (2006).
Sara, S.J. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 7, 73–84 (2000).
Nader, K. & Hardt, O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 10, 224–234 (2009).
Eisenberg, M., Kobilo, T., Berman, D.E. & Dudai, Y. Stability of retrieved memory: inverse correlation with trace dominance. Science 301, 1102–1104 (2003).
Nader, K. Memory traces unbound. Trends Neurosci. 26, 65–72 (2003).
Pedreira, M.E. & Maldonado, H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38, 863–869 (2003).
Suzuki, A. et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795 (2004).
Milekic, M.H. & Alberini, C.M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36, 521–525 (2002).
Debiec, J., Doyere, V., Nader, K. & Ledoux, J.E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl. Acad. Sci. USA 103, 3428–3433 (2006).
Stollhoff, N., Menzel, R. & Eisenhardt, D. Spontaneous recovery from extinction depends on the reconsolidation of the acquisition memory in an appetitive learning paradigm in the honeybee (Apis mellifera). J. Neurosci. 25, 4485–4492 (2005).
Duvarci, S., Mamou, C.B. & Nader, K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur. J. Neurosci. 24, 249–260 (2006).
Debiec, J., LeDoux, J.E. & Nader, K. Cellular and systems reconsolidation in the hippocampus. Neuron 36, 527–538 (2002).
Lee, J.L., Di Ciano, P., Thomas, K.L. & Everitt, B.J. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47, 795–801 (2005).
Debiec, J. & Ledoux, J.E. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–272 (2004).
DeVietti, T.L. & Holliday, J.H. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace: a replication. Psychon. Sci. 29, 137–138 (1972).
Bozon, B., Davis, S. & Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40, 695–701 (2003).
Przybyslawski, J., Roullet, P. & Sara, S.J. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J. Neurosci. 19, 6623–6628 (1999).
Kim, J.J. & Fanselow, M.S. Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992).
Ben Mamou, C., Gamache, K. & Nader, K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 9, 1237–1239 (2006).
Blanchard, R.J. & Blanchard, D.C. Crouching as an index of fear. J. Comp. Physiol. Psychol. 67, 370–375 (1969).
Rudy, J.W., Biedenkapp, J.C., Moineau, J. & Bolding, K. Anisomycin and the reconsolidation hypothesis. Learn. Mem. 13, 1–3 (2006).
Duvarci, S. & Nader, K. Characterization of fear memory reconsolidation. J. Neurosci. 24, 9269–9275 (2004).
Frankland, P.W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Frankland, P.W., Bontempi, B., Talton, L.E., Kaczmarek, L. & Silva, A.J. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883 (2004).
Zola-Morgan, S.M. & Squire, L.R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science 250, 288–290 (1990).
Blanchard, D.C., Blanchard, R.J. & Fukunaga, K.K. Environmental control of defensive reactions to footshock. Bull. Psychon. Soc. 8, 129–130 (1976).
McDonald, A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332 (1998).
Pitkanen, A., Pikkarainen, M., Nurminen, N. & Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. Ann. NY Acad. Sci. 911, 369–391 (2000).
Richter-Levin, G. & Akirav, I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol. Neurobiol. 22, 11–20 (2000).
Zinebi, F. et al. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J. Neurosci. 23, 10283–10291 (2003).
Morris, R.G. et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50, 479–489 (2006).
Fanselow, M.S. & Gale, G.D. The amygdala, fear, and memory. Ann. NY Acad. Sci. 985, 125–134 (2003).
Schafe, G.E., Nader, K., Blair, H.T. & LeDoux, J.E. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546 (2001).
Maren, S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J. Neurosci. 19, 8696–8703 (1999).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Phillips, R.G. & LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 (1992).
Ambrogi Lorenzini, C.G., Baldi, E., Bucherelli, C., Sacchetti, B. & Tassoni, G. Neural topography and chronology of memory consolidation: a review of functional inactivation findings. Neurobiol. Learn. Mem. 71, 1–18 (1999).
Moscovitch, M., Nadel, L., Winocur, G., Gilboa, A. & Rosenbaum, R.S. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 16, 179–190 (2006).
Turrigiano, G.G. & Nelson, S.B. Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol. 10, 358–364 (2000).
Franks, K.M. & Isaacson, J.S. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron 47, 101–114 (2005).
Joshi, I. & Wang, L.Y. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J. Physiol. (Lond.) 540, 861–873 (2002).
Suzuki, A., Mukawa, T., Tsukagoshi, A., Frankland, P.W. & Kida, S. Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn. Mem. 15, 426–433 (2008).
Milton, A.L., Lee, J.L., Butler, V.J., Gardner, R. & Everitt, B.J. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J. Neurosci. 28, 8230–8237 (2008).
Lee, S.H. et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319, 1253–1256 (2008).
Lengyel, I. et al. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur J. Neurosci. 20, 3063–3072 (2004).
Acknowledgements
We thank S. Kusher for detailed suggestions and comments on the manuscript. We thank various people in the lab who have helped us with these experiments. L.d.O.A. was supported by the Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The study was supported by Natural Sciences and Engineering Research Council (Canada), Canadian Institutes of Health Research, Canada Foundation for Innovation, Volkswagen Foundation and E.W.R. Steacie Memorial Fellowship.
Author information
Authors and Affiliations
Contributions
K.N. and S.-H.W. designed and developed this study. S.-H.W. conducted the behavioral, pharmacological, lesion and immunohistochemical experiments, performed the statistical and histological analyses and wrote the paper with K.N. L.d.O.A. conducted the western blot and lesion with extinction experiments and performed related data analyses. K.N. supervised this project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 889 kb)
Rights and permissions
About this article
Cite this article
Wang, SH., de Oliveira Alvares, L. & Nader, K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci 12, 905–912 (2009). https://doi.org/10.1038/nn.2350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2350
This article is cited by
-
Muscarinic receptor activation overrides boundary conditions on memory updating in a calcium/calmodulin-dependent manner
Neuropsychopharmacology (2023)
-
Demarcating the boundary conditions of memory reconsolidation: An unsuccessful replication
Scientific Reports (2022)
-
Impairment in novelty-promoted memory via behavioral tagging and capture before apparent memory loss in a knock-in model of Alzheimer’s disease
Scientific Reports (2022)
-
Sleep enhances reconsolidation-based strengthening of visuospatial memories
Scientific Reports (2022)
-
Refinement of the stress-enhanced fear learning model of post-traumatic stress disorder: a behavioral and molecular analysis
Lab Animal (2022)